Abstract
In the predator–prey arms race, survival-enhancing adaptive behaviors are essential. Prey can perceive predator presence directly from visual, auditory, or chemical cues. Non-lethal encounters with a predator may trigger prey to produce special body odors, alarm pheromones, informing conspecifics about predation risks. Recent studies suggest that parental exposure to predation risk during reproduction affects offspring behavior cross-generationally. We compared behaviors of bank vole (Myodes glareolus) pups produced by parents exposed to one of three treatments: predator scent from the least weasel (Mustela nivalis nivalis); scent from weasel-exposed voles, i.e., alarm pheromones; or a control treatment without added scents. Parents were treated in semi-natural field enclosures, but pups were born in the lab and assayed in an open-field arena. Before each behavioral test, one of the three scent treatments was spread throughout the test arena. The tests followed a full factorial design (3 parental treatments × 3 area treatments). Regardless of the parents’ treatment, pups exposed to predator odor in the arena moved more. Additionally, pups spend more time in the center of the arena when presented with predator odor or alarm pheromone compared with the control. Pups from predator odor–exposed parents avoided the center of the arena under control conditions, but they spent more time in the center when either predator odor or alarm pheromone was present. Our experiment shows that cross-generational effects are context-sensitive, depending on the perceived risk. Future studies should examine cross-generational behavioral effects in ecologically meaningful environments instead of only neutral ones.
Significance statement
We exposed bank voles to odors signaling predation risk to assess the effects parental predation exposure on the behavior of their offspring. Besides predator odor, we also assessed the role of a conspecific alarm cue as a novel way of spreading the predation risk information. Pup behaviors were assessed in the open-field arena, a standard way of assessing animal behavior in a wide range of contexts. We found that also alarm pheromone increased the time pups spend in the center of the arena similarly to predator odor. While previous studies suggested that offspring would be more fearful, our results indicate that the cross-generational effects are very context-dependent; i.e., they differ significantly depending on which scent cue is presented in the open-field arena. This shows the need for better tools or measurements to translate laboratory results into ecologically meaningful frameworks.
Similar content being viewed by others
Introduction
Predator–prey interactions are among the strongest drivers of evolution (Abrams 1986, 2000; Yoshida et al. 2003). These effects work via multiple mechanisms, e.g., from simple acute behavioral modifications to intergenerational and long-term physiological adaptations by prey animals (Abrams 2000). Thus, in the context of an evolutionary arms race, early recognition of predation risk by prey is of paramount importance for their survival. For instance, when small mammals and raptors interact, antipredation tactics used by the prey include reliance on visual predator cues and avoidance of open areas where aerial predators can hunt most effectively (Lima and Dill 1990; Kotler et al. 1991). In predator–prey interactions between mammals, olfactory cues (and the associated sensory abilities) are more important. These cues and senses are critical to predators and prey in locating and avoiding each other in the covered or enclosed habitats that they share, including tunnels and cavities where the animals live or nest (Haapakoski and Ylönen 2013; Haapakoski et al. 2013; Ylönen et al. 2019).
The different types of cues that prey use to monitor the presence of predators can be transmitted directly or indirectly. Direct cues originate from predators. An attack is probably the most unambiguous direct cue, but predator scents also qualify. Indirect cues originate from other prey and include warning calls (Townsend et al. 2012; Collier et al. 2017), other behavioral changes (Graw and Manser 2007; Cornell et al. 2012), and alarm secretions (Verheggen et al. 2010). Indirect cues are typically the result of direct ones. For example, if a prey animal survives a predator encounter or attack, then the fear response can result in the prey producing olfactory signals, or alarm pheromones, which can alert conspecifics about predator presence (Breed et al. 2004; Kiyokawa et al. 2004). The antipredatory responses of the recipients of these indirect scent cues are expected to resemble the responses of conspecifics receiving olfactory cues directly from predators. This hypothesis is supported by recent results of behavioral and physiological studies: for example, alarm cue–induced avoidance responses (Gomes et al. 2013), analgesic responses (Kavaliers et al. 2005), and changes in reproduction (Haapakoski et al. 2018). The similar effects of indirect and direct scent cues may result from biochemical similarities between the alarm pheromones produced by prey and scents produced by predators, as reported by Brechbühl et al. (2013).
An area of research on predator–prey interactions that remains relatively unexplored in recent years relates to the transmission of information about predation risk from prey parent to offspring. Specifically, the extent to which information on predation risk is transferred by predation cue recipient mothers to their in utero offspring, i.e., cross-generational effects via in utero imprinting did not receive much attention over the last decade, but is again gaining traction with researchers. The earliest work in this area demonstrated that direct exposure (i.e., via injection into the amniotic sac) to a chemical cue in utero can be used for imprinting/conditioning of the offspring (Smotherman 1982a, b; Stickrod et al. 1982). In general, cross-generational effects could mean that pups whose mothers experienced higher than normal predation risks exhibit different development or antipredator behaviors compared with pups whose mothers experienced low risk. Mothers that were chased or even attacked by a predator while gravid might also produce pups that develop or behave differently.
A possible mechanism for such cross-generational effects is maternal hormones, whose production can be altered by stressful events. Maternal hormones can exert an in utero influence on the physiology and possibly the behavior and life history traits of pups later in life (Caldji et al. 1998; Love and Williams 2008; Sheriff and Love 2013). If unborn pups are exposed to hormones related to stress caused by high predation risk, then the pups’ behavioral responses to that stressor can be programmed differently in utero compared with unexposed pups. Such effects have been demonstrated in response to a wide variety of external stimuli, including foot-shocks (Archer 1973), impoverished environments (Dell and Rose 1987), and scent cues (Champagne and Meaney 2006). Much less is known regarding the role of paternal factors, but Rodgers et al. (2013) found that lifelong paternal experience can induce germ cell epigenetic reprogramming and impact offspring HPA stress axis regulation in mice.
In this study, we compared the cross-generational effects of predation risk on pup behavior using three different scent cues: predator odor (PO), scent of frightened conspecifics (alarm pheromone, AP), and an “unscented” control with no added scent (C). Parents living in semi-natural field enclosures were exposed to the scent cues individually. Mothers were brought to the lab to give birth, and at about 5 weeks of age, pups were behaviorally assayed in an open-field arena containing the PO, AP, or C cue. We expected that if parental exposure to cues of predation risk has cross-generational effects, then we would see differences between the parental treatments regardless of the pup treatment in the open-field arena. More specifically, we expected the combined parental–pup exposure to predator odor (i.e., PO-PO) would decrease pup exploration behavior, as measured by movement in the open-field arena, more than either PO-C or C-PO (Norrdahl and Korpimäki 1998). Decreased exploration behavior would indicate a greater antipredator response (Norrdahl and Korpimäki 1998). Should there be a difference in the effect sizes between the two experimental scent cues, then we expected PO would exert stronger effects than AP.
Material and methods
Study animals
The bank vole (Myodes glareolus) is one of the most common small rodents living in a variety of northern temperate and boreal European forest habitats west of the Urals (Stenseth 1985). The species is granivorous-omnivorous, with their diet consisting mainly of seeds and buds, and also of other plant materials or invertebrates (Hansson 1979; Eccard and Ylönen 2006). The gestation period of the bank vole is 19–20 days, and the weaning period is 3 weeks. Litter size averages 5–6 pups but ranges between 2 and 10. In Central Finland, where this work was conducted, bank voles breed three to five times per season, which lasts from May until September (Mappes et al. 1995; Koivula et al. 2003). Bank voles are preyed upon by a diverse predator assemblage, including least weasels (Mustela nivalis) and stoats (Mustela erminea) (Ylönen 1989; Meri et al. 2008). The least weasel is an especially effective hunter of voles due to their size and excellent hunting skills; least weasels are likely able to kill bank voles whenever the two species come into direct contact (Tidhar et al. 2007; Haapakoski et al. 2012).
We conducted our study at the Konnevesi Research Station in Central Finland (62° 37′ N, 26° 20′ E). In the laboratory, voles were housed in husbandry rooms under a 16L:8D light regime with a constant temperature (22 °C ± 1 °C); males and females were maintained in the same room. All animals were kept individually in 42 cm × 26 cm × 15 cm transparent cages with wire mesh lids and supplied with ad libitum water and food. The bedding materials in each cage consisted of wood shavings and hay. The breeding adults used in the study were the F1 generation of wild-caught individuals that were housed in the lab during the winter months preceding the study period. Winter colonies are formed from the last cohort of voles of the previous summer. Thus, their age when paired for the first breeding is around 7 months. The winter population is housed on a short photoperiod (8L:16D) at around 17 °C throughout the winter and male voles’ testes are abdominal and female vaginas are closed. Only after adjusting the photoperiod to long day in spring to prepare for breeding, our voles become reproductive again. This is done starting from February when the first voles start to breed also in the field (Haapakoski et al. 2012). All animals were individually marked with ear tags (#1005-1L1, National Band & Tag Company, Newport, KY, USA).
Weasels for the scent treatment were housed individually in 60 cm × 160 cm × 60 cm cages in an outdoor shelter. Each cage had a nest box and wood shavings and hay as bedding. Throughout the experiment (and during the 2-week period before its initiation), weasels were exclusively fed dead bank voles.
Scent cues
The predator odor (PO) was obtained by collecting used bedding materials, which included feces, urine, and body odor, from the three captive least weasels. The odor cues were always used within 1 h after being taken from the weasel cage. No odor cue was stored for later use. The alarm pheromone cue (AP) was obtained by collecting bedding materials that were used by male bank voles that were exposed to the weasels on a daily basis. During the exposure, AP “donor voles” were placed inside a wire mesh live trap, which was placed inside the weasel cage for 60 s. After the initial exposure, donor voles were placed individually in clean cages with fresh bedding materials to allow their scents to infuse into the bedding materials. The scents produced by the weasel-exposed voles were allowed to accumulate in used bedding materials following successive exposures. Bedding materials were only used as an AP cue after at least three consecutive exposures. The control odor cue (C) was clean bedding materials without any added cues from voles or weasels. In order to minimize variation in odor source, the beddings were thoroughly mixed before taking samples of bedding with urine and/or fecal matter. The same procedure was used for the clean bedding.
Field phase
Fieldwork was conducted using nine 0.25-ha outdoor enclosures that are part of the Konnevesi Research Station in Central Finland (Haapakoski et al. 2012). The enclosures, which were emptied of other rodents by live trapping before the experiment was started, were split evenly among the three treatment groups. Each enclosure contained 25 multiple-capture live traps (Ugglan Special, Grahnab AB, Gnosjö, Sweden) arranged in a 5 × 5 grid with 10-m intervals between traps. The traps were placed inside lidded metal chimneys to protect captured individuals from exposure to the elements.
In mid-June, six females were released in each enclosure, allowing them to establish territories. Two days later, four males were added to each enclosure. After a 1-day acclimation period following the addition of the males, the traps were activated and checked two times per day. Thus, a total of 54 female and 36 male voles, all of which were 11–13 months old, were used in the field.
For every capture event throughout the full 14-day experimental period, the ID of each captured vole was recorded, and captured individuals of both sexes were exposed to scent cues according to the treatment randomly assigned to their enclosure at the start of the experiment. The voles were presented with scent cues in a treatment chamber, a tightly closing box made of plywood with a perforated divider (i.e., a plastic wall with holes; see Suppl. material Fig. A). Two dl of PO, AP, or C bedding materials was placed on one side of the divider, an individual vole in a live trap was placed on the other side, and the chamber was closed for 3 min. Immediately after the treatment, voles were released at the site of capture. The odor cues, each in their own dedicated treatment chamber, were renewed twice per day. Recapture rate was similar in each treatment (see “Results” for more details).
From day 15 onwards, captured voles were removed from the enclosure and brought back to the laboratory where the pregnant females gave birth.
Laboratory phase
In the laboratory, all females were checked daily for parturition from day 17 onwards. The pups were weaned, separated, sexed, and when 23 days old, ear-tagged. Pups were then caged individually in separate husbandry rooms, as described above, according to their parents’ experimental treatment.
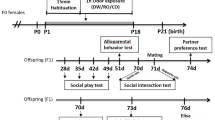
Pups from each parental treatment group (PO, AP, C) were assigned to one of three pup treatment groups (also PO, AP, or C; Fig. 1) to study cross-generational effects on behavior. Pups from the same mother were distributed among treatment groups; the sex ratios in each parental-pup treatment combination were kept similar. Once over 30 days of age (37.7 ± 5.5 days, mean ± standard deviation), pups were behaviorally assayed on an individual basis in an open-field arena, which allowed for easy measurement for fearfulness and vigilance in exploratory behavior. Immediately prior to assaying a pup, 1 dl of bedding materials corresponding to the pup’s treatment was spread equally throughout the arena. The arena was separated into a border zone (a total of 20 10 × 10 cm squares) and a center zone (a total of sixteen 10 × 10 cm squares; Suppl. material Fig. B). After a pup was released in the central circle of the arena, its behavior was filmed from above for 10 min with a GoPro 4 camera. After each trial, the bedding was removed, and the arena was thoroughly cleaned with 70% alcohol and allowed to dry.
Experimental design showing parental and pup treatments. All assayed pups experienced a parental treatment via their mothers (in utero) exposure and a pup treatment directly in the open-field arena: C, control bedding; AP, alarm pheromone treatment with weasel-exposed bank vole bedding; and PO, predation odor treatment with weasel bedding
Video analysis was done automatically with the EthoVision XT (Noldus et al. 2001) and overseen by a single researcher (AK). In the program, the different zones were marked, and the settings were calibrated separately per video. Movement frequency between the center zone and the border zone, total distance moved, time spent in the border zone, and movement bouts were measured automatically. A movement bout was defined as constant movement with less than 5 s of no movement. If an individual remained still for ≥ 5 s, a new bout was recorded.
It was not possible to record data blind because our study involved scent cues, which are easily visually or by scent distinguishable.
Data analysis
All statistical analyses were performed in R (R Core Team 2019), and figures were produced using “ggplot2” (Wickham 2016) and “ggsignif” (Ahlmann-Eltze 2019). Litter sizes were analyzed using a zero-augmented model in “pscl” (Zeileis et al. 2008). To analyze the potential effect of the treatments on the recapture rate per day in the field, we used the package “marked” (Laake et al. 2013). A recapture rate exceeding 100% was possible because we trapped twice per day. Two variables from the laboratory-based behavioral assay, total distance moved (cm) and time spent (s) in the border zone, were analyzed with a linear mixed model (LMM) using “lme4” (Bates et al. 2015) and “lmertest” (Kuznetsova et al. 2017). The third behavioral variable, proportion of time in center (time in center: total time), was analyzed with a generalized linear mixed model (GLMM) with a binomial distribution using “lme4” (Bates et al. 2015). The independent variables included in models examining cross-generational effects were parental treatment, pup treatment, pup sex, litter size, and pup age during the test. The initial model contained the three-way interaction of parental treatment × pup treatment × pup sex. Additionally, the ID of the mother was included as a random factor. Non-significant interactions were discarded one by one, always starting with the three-way interaction of parental treatment × pup treatment × pup sex. The two-way interaction of parental treatment × pup treatment was always the last to be removed because this interaction was central to our research question. The full model and all models that resulted from the backwards selection procedure were ranked by AICc. If a single model was identified as best (i.e., > 2 AICc units from second best), then this model was used when reporting the significance of explanatory variables. If more than one model were identified as best (i.e., within 2 AICc units of the best model), the average p values and model estimates, weighted by AICc, are reported. AICc assessment and model averages are obtained with “MuMIn” (Barton 2019) (more detailed information about the model selection procedure is provided in the supplemental material (Tables S1-S5 and R code)).
Results
Parents
The recapture rate of adults in the field enclosures did not differ among odor treatments but did differ between sexes. Males had a recapture probability of 116% ± 15% per day; females had a recapture probability of 54% ± 16% per day (weighted average ± weighted standard error by AICc). For four of 24 models, ΔAIC ≤ 2 (Suppl. material, Table S1). All four models included sex as a factor, and only one included treatment as a factor. The combined additive weight by AICc of the models not containing the treatment is 0.55, whereas the model containing the treatment has a weight of 0.17.
Thirty-one of 54 females were found to be pregnant: 11 C (sired 4.72 ± 1.27 pups, mean ± standard deviation), 10 AP (5.2 ± 1.14), and 10 PO (6.1 ± 1.1). Litter sizes did not differ significantly by treatment (n = 31, df = 6, PO vs. C p = 0.579, AP vs. C p = 0.142).
Pups
In total, 135 pups were tested in the open-field arena. The total distance moved differed among pup treatments (LMM; n = 135; Table S2, p < 0.001, weighted average by AICc); PO pups moved significantly more than C pups (about 998 cm; weighted model average by AICc; Fig. 2). Parental treatment and litter size were not included in the best models based on AICc.
Total distance moved in the open-field arena by treatment during a 10-min period. Three asterisks indicate a significant difference from C (p < 0.001). Each boxplot consists of the following elements: median value (bold horizontal line), 0.75 and 0.25 percentiles (upper and lower box limits, respectively), and the highest and lowest values (upper and lower whisker ends, respectively). The boxplot whiskers are limited to 1.5 times the interquartile range. Outliers are marked as dots
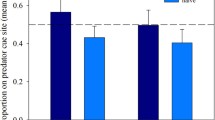
The proportion of time pups spent in the central zone of the open-field arena differed significantly depending on both parental and pup treatments separately (GLMM, n = 135, df = 12, Table S3; Fig. 3). Pups from control parents exposed to control bedding materials in the arena (i.e., C/C, parental/F1) spent about 18% of the time in the center zone. Some pups spent significantly less time in the center zone (C/PO decreased by 12%; p = 0.012); some spent significantly more (PO/C increased by 25%; p < 0.0001 and AP/C increased by 21%; p = 0.0017; all results from GLMM above). Aside from the single effects of the treatments, the interaction resulted for PO/PO in 22% of time spent in the center zone (p < 0.0001, the same GLMM as above). Similarly, AP/PO spent 26% of the time in the center (p < 0.0001, the same GLMM as above). Additionally, regardless of treatment, females spent 6% less time in the center zone than males (p = 0.0099, the same GLMM as above), and older pups spent less time in the center than younger ones (by about 3% per day; p < 0.0001, the same GLMM as above).
Proportion of time in the center zone of the open-field arena during a 10-min period. Columns show the parental treatment; rows show the offspring (F1) treatment in the open-field arena. One asterisk indicates significance at p < 0.05, two asterisks at p < 0.01, and three asterisks at p < 0.001. All comparisons are with C/C. The dotted line indicates the C/C value and serves to aide in comparisons. All model averages (dots), weighted by AICc, are shown with their respective 95% confidence intervals (whiskers)
Lastly, the time spent in the border zone correlated negatively and significantly with the number of movement bouts in that zone (LMM, n = 135, Table S4; all models p < 0.0001; Fig. 4), while the time spent in the center zone correlated positively and significantly with the number of movement bouts in that zone (LMM, n = 135, Table S5; all models p < 0.0001; Fig. 4). For each movement bout in the border, the time spent in the border zone was 1.4 s less; for each movement bout in the center zone, the time spent in the center zone increased by 2.5 s (both by weighted average).
Discussion
We examined the extent to which the predation experiences of parents are transmitted cross-generationally, via in utero imprinting, to their pups. Further, we examined how the environment of the pups influences the manifestation of any behavioral effects of imprinting. We did this by first exposing parents and then subsequently their offspring to one of three scent treatments (direct predator odor, PO; indirect alarm pheromone, AP; or control, C) in a full factorial design and by testing the behavior of pups in an open-field arena. We found cross-generational effects: pups from predator odor–exposed parents sought shelter along the edges of the arena when exposed to the control cue, but pups from that same parental exposure group took more risks by spending more time in the center of the arena when exposed to predator odor or alarm pheromone.
Contrary to our hypothesis based on previous research (Jędrzejewski et al. 1993; Norrdahl and Korpimäki 1998), pups exposed to predator odor during behavioral testing, regardless of their in utero treatment, did not decrease their total distance moved. In general, prey animals like voles are expected to make use of safe places within their broader environment to reduce their chances of encountering and being attacked by a predator. For example, prey can flee to a safe place to avoid a predator (Mäkeläinen et al. 2014), or they can decrease movement or even freeze in place if they are already in a safe place and predators are in the vicinity (Sundell and Ylönen 2004; Apfelbach et al. 2005). In our open-field arena, retreat to a safe place was not possible since the risk-cue was evenly distributed throughout and no hiding places or refuges were offered, which both could help explain our results.
The pups exposed to the experimental scent cues during testing may have kept moving to avoid encountering a predator (Jędrzejewski et al. 1993), but they also could have been gathering information about the odor landscape in an attempt to identify a relatively safe place (Parsons et al. 2018). In other studies (Jędrzejewski et al. 1993; Norrdahl and Korpimäki 1998), safety was offered in the form of hiding places. However, because exposure to alarm pheromone treatment during behavioral testing did not elicit the same effects as the predator odor treatment, behavioral responses to predator cues can depend solely on scent traits and olfaction and do require the activation of other senses. This might raise the question whether the method to collect AP is suitable. However, studies using a similar method (Haapakoski et al. 2018; Sievert et al. 2019) clearly showed significant changes in voles when presented with the AP cue.
Antipredator behavior is extremely complex, and split-second responses of animals may make the difference between life and death. For these reasons, an open-field arena test is certainly a simplification of the natural situation. Nevertheless, spending more time away from the center of an open-field arena is commonly interpreted as a consequence (and therefore a proxy) of anxiety (Treit and Fundytus 1988). In our study, pups exhibited significant cross-generational differences in the proportion of time spent in the center zone. While treating parents with predator odor resulted in their offspring exhibiting increased anxiety-like behavior in the control testing environment, the combination of prenatal predator odor with either predator odor or alarm pheromone in the testing environment resulted in offspring reacting less fearfully. Thus, our results indicate that cross-generational changes in behavior are highly context-dependent, while simultaneously supporting earlier cross-generational studies that documented higher levels of anxiety-like behavior when offspring of stressed parents are tested in neutral environments (Abe et al. 2007; Brunton and Russell 2010; Brunton 2013; St-Cyr et al. 2017). Cross-generational effects have been proposed to act as an adaptive bridge between the maternal and offspring environment (Love et al. 2013). Our study supports this view.
Increased boldness in a high-risk environment, as a result of in utero imprinting, might be adaptive. Being bold might equate to a greater willingness to explore, forage, and mate instead of waiting for the risk to abate (Ylönen et al. 2002; Korpela et al. 2011; Mella et al. 2015). In fact, over the lifespan of a short-lived rodent, predation pressure is unlikely to decline rapidly. Predator density is known to track prey density with a time lag, and this lag can mean a high predator-to-prey ratio lasts longer than the prey generation time (Hanski et al. 2001; Sundell et al. 2013).
Our experiment was designed so that the prey voles can encounter fresh predator cues daily. This design represents a situation with a high predator-to-prey ratio, such as the case when vole populations are declining. Small mammal populations fluctuate cyclically in boreal landscapes (e.g., Hanski et al. 2001), and weasel populations follow the vole density with an approximately half-year time lag (Korpimäki et al. 1991). Independently of population cycles, weasel densities during summer in deciduous forest can approach 4.5 individuals/km2 (Jędrzejewski et al. 1995). Under such densities, voles likely frequently encounter weasels or signs of weasel presence, and this encounter rate is further elevated by female weasels nesting in the vicinity of voles. Finally, in addition to weasels, voles may encounter other small mustelid predators such as stoats (Erlinge et al. 1982) and pine martens (Martes martes), both of which produce distinctive mustelid odors (Brinck et al. 1983).
Weasel movement ecology and hunting efficiency have proven hard to study experimentally, but Norrdahl and Korpimäki (1995) estimated that weasels and stoats account for almost 80% of the total mortality for three vole species during the period of vole population decline. Though weasels are effective lethal predators, voles are well adapted to weasel presence and evasion. In a radio-tracking study in the same enclosures as used for the current experiment, we observed a clear relationship between weasel inactivity and vole activity. When weasels were resting, voles were active, regardless of the time of the day or night (Sundell et al. 2008). In addition to temporal avoidance of predation risk, bank voles also use arboreal escape routes when weasels are giving chase (Mäkeläinen et al. 2014). These different survival strategies represent scenarios in which prey voles are expected to produce AP.
Our study focused on cross-generational effects of different scent-based predation cues on offspring behavior. Using behavior as a starting point to understand possible fitness effects, our results highlight the need for future studies to test this type of cross-generational behavioral effect in pups living in or exposed to different environmental conditions. Ultimately, the goal should be to investigate the interaction of pre- and post-natal cues about predator pressure on reproduction and survival in ecologically meaningful instead of neutral environments.
Data availability
The datasets analyzed during the current study are available in the figshare repository, www.doi.org/10.6084/m9.figshare.8057303
The R code to analyze the data is available in the figshare repository. www.doi.org/10.6084/m9.figshare.8057276
References
Abe H, Hidaka N, Kawagoe C, Odagiri K, Watanabe Y, Ikeda T, Ishizuka Y, Hashiguchi H, Takeda R, Nishimori T, Ishida Y (2007) Prenatal psychological stress causes higher emotionality, depression-like behavior, and elevated activity in the hypothalamo-pituitary-adrenal axis. Neurosci Res 59:145–151. https://doi.org/10.1016/j.neures.2007.06.1465
Abrams PA (1986) Is predator-prey coevolutlon an arms race? Trends Ecol Evol 1:108–110. https://doi.org/10.1016/0169-5347(86)90037-6
Abrams PA (2000) The evolution of predator-prey interactions: theory and evidence. Annu Rev Ecol Syst 31:79–105. https://doi.org/10.1146/annurev.ecolsys.31.1.79
Ahlmann-Eltze (2019). ggsignif: Significance Brackets for ‘ggplot2’. R package version 0.6.0, https://CRAN.R-project.org/package=ggsignif
Apfelbach R, Blanchard CD, Blanchard RJ, Hayes RA, McGregor IS (2005) The effects of predator odors in mammalian prey species: a review of field and laboratory studies. Neurosci Biobehav Rev 29:1123–1144. https://doi.org/10.1016/j.neubiorev.2005.05.005
Archer J (1973) Tests for emotionality in rats and mice: a review. Anim Behav 21:205–235. https://doi.org/10.1016/S0003-3472(73)80065-X
Kamil Barton (2019) MuMIn: Multi-Model Inference. R package version 1.43.6, https://CRAN.R-project.org/package=MuMIn
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Brechbühl J, Moine F, Klaey M, Nenniger-Tosato M, Hurni N, Sporkert F, Giroud C, Broillet M-C (2013) Mouse alarm pheromone shares structural similarity with predator scents. P Natl Acad Sci USA 110:4762–4767. https://doi.org/10.1073/pnas.1214249110
Breed MD, Guzmán-Novoa E, Hunt GJ (2004) Defensive behavior of honey bees: organization, genetics, and comparisons with other bees. Annu Rev Entomol 49:271–298. https://doi.org/10.1146/annurev.ento.49.061802.123155
Brinck C, Erlinge S, Sandell M (1983) Anal sac secretion in mustelids a comparison. J Chem Ecol 9:727–745. https://doi.org/10.1007/BF00988779
Brunton PJ (2013) Effects of maternal exposure to social stress during pregnancy: consequences for mother and offspring. Reproduction 146:R175–R189. https://doi.org/10.1530/REP-13-0258
Brunton PJ, Russell JA (2010) Prenatal social stress in the rat programmes neuroendocrine and behavioural responses to stress in the adult offspring: sex-specific effects. J Neuroendocrinol 22:258–271. https://doi.org/10.1111/j.1365-2826.2010.01969.x
Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ (1998) Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. P Natl Acad Sci USA 95:5335–5340. https://doi.org/10.1073/pnas.95.9.5335
Champagne FA, Meaney MJ (2006) Stress during gestation alters postpartum maternal care and the development of the offspring in a rodent model. Biol Psychiatry 59:1227–1235. https://doi.org/10.1016/j.biopsych.2005.10.016
Collier K, Radford AN, Townsend SW, Manser MB (2017) Wild dwarf mongooses produce general alert and predator-specific alarm calls. Behav Ecol 28:1293–1301. https://doi.org/10.1093/beheco/arx091
Cornell HN, Marzluff JM, Pecoraro S (2012) Social learning spreads knowledge about dangerous humans among American crows. Proc R Soc Lond B 279:499–508. https://doi.org/10.1098/rspb.2011.0957
Dell PA, Rose FD (1987) Transfer of effects from environmentally enriched and impoverished female rats to future offspring. Physiol Behav 39:187–190. https://doi.org/10.1016/0031-9384(87)90008-4
Eccard JA, Ylönen H (2006) Adaptive food choice of bank voles in a novel environment: choices enhance reproductive status in winter and spring. Ann Zool Fenn 43:2–8
Erlinge S, Sandell M, Brinck C (1982) Scent-marking and its territorial significance in stoats, Mustela erminea. Anim Behav 30:811–818. https://doi.org/10.1016/S0003-3472(82)80154-1
Gomes LAP, Salgado PMP, Barata EN, Mira APP (2013) Alarm scent-marking during predatory attempts in the Cabrera vole (Microtus cabrerae Thomas, 1906). Ecol Res 28:335–343. https://doi.org/10.1007/s11284-012-1023-8
Graw B, Manser MB (2007) The function of mobbing in cooperative meerkats. Anim Behav 74:507–517. https://doi.org/10.1016/j.anbehav.2006.11.021
Haapakoski M, Ylönen H (2013) Snow evens fragmentation effects and food determines overwintering success in ground-dwelling voles. Ecol Res 28:307–315. https://doi.org/10.1007/s11284-012-1020-y
Haapakoski M, Sundell J, Ylönen H (2012) Predation risk and food: opposite effects on overwintering survival and onset of breeding in a boreal rodent: predation risk, food and overwintering. J Anim Ecol 81:1183–1192. https://doi.org/10.1111/j.1365-2656.2012.02005.x
Haapakoski M, Sundell J, Ylönen H (2013) Mammalian predator–prey interaction in a fragmented landscape: weasels and voles. Oecologia 173:1227–1235. https://doi.org/10.1007/s00442-013-2691-y
Haapakoski M, Hardenbol AA, Matson KD (2018) Exposure to chemical cues from predator-exposed conspecifics increases reproduction in a wild rodent. Sci Rep 8:17214. https://doi.org/10.1038/s41598-018-35568-0
Hanski I, Henttonen H, Korpimäki E, Oksanen L, Turchin P (2001) Small-rodent dynamics and predation. Ecology 82:1505–1520. https://doi.org/10.2307/2679796
Hansson L (1979) Condition and diet in relation to habitat in bank voles Clethrionomys glareolus: population or community approach? Oikos 33:55–63. https://doi.org/10.2307/3544511
Jędrzejewski W, Rychlik L, Jędrzejewska B (1993) Responses of bank voles to odours of seven species of predators: experimental data and their relevance to natural predator-vole relationships. Oikos 68:251–257. https://doi.org/10.2307/3544837
Jędrzejewski W, Jędrzejewska B, Szymura L (1995) Weasel population response, home range, and predation on rodents in a deciduous forest in Poland. Ecology 76:179–195. https://doi.org/10.2307/1940640
Kavaliers M, Choleris E, Pfaff DW (2005) Recognition and avoidance of the odors of parasitized conspecifics and predators: differential genomic correlates. Neurosci Biobehav Rev 29:1347–1359. https://doi.org/10.1016/j.neubiorev.2005.04.011
Kiyokawa Y, Kikusui T, Takeuchi Y, Mori Y (2004) Alarm pheromones with different functions are released from different regions of the body surface of male rats. Chem Senses 29:35–40. https://doi.org/10.1093/chemse/bjh004
Koivula M, Koskela E, Mappes T, Oksanen TA (2003) Cost of reproduction in the wild: manipulation of reproductive effort in the bank vole. Ecology 84:398–405. https://doi.org/10.1890/0012-9658(2003)084[0398:CORITW]2.0.CO;2
Korpela K, Sundell J, Ylönen H (2011) Does personality in small rodents vary depending on population density? Oecologia 165:67–77. https://doi.org/10.1007/s00442-010-1810-2
Korpimäki E, Norrdahl K, Rinta-Jaskari T (1991) Responses of stoats and least weasels to fluctuating food abundances: is the low phase of the vole cycle due to mustelid predation? Oecologia 88:552–561. https://doi.org/10.1007/BF00317719
Kotler BP, Brown JS, Hasson O (1991) Factors affecting gerbil foraging behavior and rates of owl predation. Ecology 72:2249–2260. https://doi.org/10.2307/1941575
Kuznetsova A, Brockhoff PB, Christensen RHB (2017) lmerTest package: tests in linear mixed effects models. J Stat Softw 82:1–26. https://doi.org/10.18637/jss.v082.i13
Laake JL, Johnson DS, Conn PB (2013) Marked: an R package for maximum likelihood and Markov chain Monte Carlo analysis of capture-recapture data. Methods Ecol Evol 4:885–890. https://doi.org/10.1111/2041-210X.12065
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640. https://doi.org/10.1139/z90-092
Love OP, Williams TD (2008) The adaptive value of stress-induced phenotypes: effects of maternally derived corticosterone on sex-biased investment, cost of reproduction, and maternal fitness. Am Nat 172:E135–E149. https://doi.org/10.1086/590959
Love OP, McGowan PO, Sheriff MJ (2013) Maternal adversity and ecological stressors in natural populations: the role of stress axis programming in individuals, with implications for populations and communities. Funct Ecol 27:81–92. https://doi.org/10.1111/j.1365-2435.2012.02040.x
Mäkeläinen S, Trebatická L, Sundell J, Ylönen H (2014) Different escape tactics of two vole species affect the success of the hunting predator, the least weasel. Behav Ecol Sociobiol 68:31–40. https://doi.org/10.1007/s00265-013-1619-1
Mappes T, Koskela E, Ylönen H (1995) Reproductive costs and litter size in the bank vole. Proc R Soc Lond B 261:19–24. https://doi.org/10.1098/rspb.1995.0111
Mella VSA, Ward AJW, Banks PB, McArthur C (2015) Personality affects the foraging response of a mammalian herbivore to the dual costs of food and fear. Oecologia 177:293–303. https://doi.org/10.1007/s00442-014-3110-8
Meri T, Halonen M, Mappes T, Suhonen J (2008) Younger bank voles are more vulnerable to avian predation. Can J Zool 86:1074–1078. https://doi.org/10.1139/Z08-087
Noldus LPJJ, Spink AJ, Tegelenbosch RAJ (2001) EthoVision: a versatile video tracking system for automation of behavioral experiments. Behav Res Meth Instr 33:398–414. https://doi.org/10.3758/BF03195394
Norrdahl K, Korpimäki E (1995) Mortality factors in a cyclic vole population. Proc R Soc Lond B 261:49–53. https://doi.org/10.1098/rspb.1995.0116
Norrdahl K, Korpimäki E (1998) Does mobility or sex of voles affect risk of predation by mammalian predators? Ecology 79:226–232. https://doi.org/10.2307/176877
Parsons MH, Apfelbach R, Banks PB et al (2018) Biologically meaningful scents: a framework for understanding predator-prey research across disciplines. Biol Rev 93:98–114. https://doi.org/10.1111/brv.12334
R Core Team (2019) R: A language and environment for statistical computing, R version 3.6.1. R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org/
Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL (2013) Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J Neurosci 33:9003–9012. https://doi.org/10.1523/JNEUROSCI.0914-13.2013
Sheriff MJ, Love OP (2013) Determining the adaptive potential of maternal stress. Ecol Lett 16:271–280. https://doi.org/10.1111/ele.12042
Sievert T, Haapakoski M, Palme R, Voipio H, Ylönen H (2019) Secondhand horror: effects of direct and indirect predator cues on behavior and reproduction of the bank vole. Ecosphere 10:e02765. https://doi.org/10.1002/ecs2.2765
Smotherman WP (1982a) In utero chemosensory experience alters taste preferences and corticosterone responsiveness. Behav Neural Biol 36:61–68. https://doi.org/10.1016/S0163-1047(82)90245-X
Smotherman WP (1982b) Odor aversion learning by the rat fetus. Physiol Behav 29:769–771. https://doi.org/10.1016/0031-9384(82)90322-5
St-Cyr S, Abuaish S, Sivanathan S, McGowan PO (2017) Maternal programming of sex-specific responses to predator odor stress in adult rats. Horm Behav 94:1–12. https://doi.org/10.1016/j.yhbeh.2017.06.005
Stenseth NC (1985) Geographic distribution of Clethrionomys species. Ann Zool Fenn 22:215–219
Stickrod G, Kimble DP, Smotherman WP (1982) In utero taste/odor aversion conditioning in the rat. Physiol Behav 28:5–7. https://doi.org/10.1016/0031-9384(82)90093-2
Sundell J, Ylönen H (2004) Behaviour and choice of refuge by voles under predation risk. Behav Ecol Sociobiol 56:263–269. https://doi.org/10.1007/s00265-004-0777-6
Sundell J, Trebatická L, Oksanen T, Ovaskainen O, Haapakoski M, Ylönen H (2008) Predation on two vole species by a shared predator: antipredatory response and prey preference. Popul Ecol 50:257–266. https://doi.org/10.1007/s10144-008-0086-4
Sundell J, O’Hara RB, Helle P, Hellstedt P, Henttonen H, Pietiäinen H (2013) Numerical response of small mustelids to vole abundance: delayed or not? Oikos 122:1112–1120. https://doi.org/10.1111/j.1600-0706.2012.00233.x
Tidhar W, Bonier F, Speakman JR (2007) Sex- and concentration-dependent effects of predator feces on seasonal regulation of body mass in the bank vole Clethrionomys glareolus. Horm Behav 52:436–444. https://doi.org/10.1016/j.yhbeh.2007.06.009
Townsend SW, Rasmussen M, Clutton-Brock T, Manser MB (2012) Flexible alarm calling in meerkats: the role of the social environment and predation urgency. Behav Ecol 23:1360–1364. https://doi.org/10.1093/beheco/ars129
Treit D, Fundytus M (1988) Thigmotaxis as a test for anxiolytic activity in rats. Pharmacol Biochem Behav 31:959–962. https://doi.org/10.1016/0091-3057(88)90413-3
Verheggen FJ, Haubruge E, Mescher MC (2010) Alarm pheromones—chemical signaling in response to danger. Vitam Horm 83:215–239
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer-Verlag, New York
Ylönen H (1989) Weasels Mustela nivalis suppress reproduction in cyclic bank voles Clethrionomys glareolus. Oikos 55:138–140. https://doi.org/10.2307/3565886
Ylönen H, Jacob J, Davies MJ, Singleton GR (2002) Predation risk and habitat selection of Australian house mice, Mus domesticus, during an incipient plague: desperate behaviour due to food depletion. Oikos 99:284–289. https://doi.org/10.1034/j.1600-0706.2002.990208.x
Ylönen H, Haapakoski M, Sievert T, Sundell J (2019) Voles and weasels in the boreal Fennoscandian small mammal community: what happens if the least weasel disappears due to climate change? Integr Zool 14:327–340. https://doi.org/10.1111/1749-4877.12388
Yoshida T, Jones LE, Ellner SP, Fussmann GF, Hairston NG (2003) Rapid evolution drives ecological dynamics in a predator–prey system. Nature 424:303–306. https://doi.org/10.1038/nature01767
Zeileis A, Kleiber C, Jackman S (2008) Regression models for count data in R. J Stat Softw 27:1–25. https://doi.org/10.18637/jss.v027.i08
Acknowledgments
We would like to thank Teemu Käpylä and Helinä Voipio for helping in the lab and the technical staff at the Konnevesi Research station for their support. We would also like to thank two anonymous reviewers for their helpful and constructive comments on the manuscript.
Funding
Open access funding provided by University of Jyväskylä (JYU). The study has been funded by an Academy of Finland grant awarded to HY (no. 288990, 11.5.2015).
Author information
Authors and Affiliations
Contributions
TS, AK, MH, KM, and HY designed the study. TS, AK, and OY collected data. TS, AK, MH, KM, and HY were involved in writing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All experiments were performed according to applicable European, national and institutional guidelines. Procedures were conducted according to national and institutional ethical standards (permit ESAVI/6370/04.10.07/2014). Keeping weasels in captivity for experimental use was done under the permission KESELY/2022/2015.
Additional information
Communicated by A. I. Schulte-Hostedde
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 98.6 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sievert, T., Kerkhoven, A., Haapakoski, M. et al. In utero behavioral imprinting to predation risk in pups of the bank vole. Behav Ecol Sociobiol 74, 13 (2020). https://doi.org/10.1007/s00265-019-2791-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-019-2791-8