Abstract
Many bird populations are made up of social units with differences in size and social status. Of these, the family and flock structure of geese Anserini are among the better known. However, how the association of juvenile geese with their parents in families influences the migration timing and space-use of populations, as well as the events leading to juvenile independence are not well understood. We focus on family size dynamics of the Greater White-fronted Goose Anser a. albifrons on its wintering grounds in the Netherlands and northern Germany, where we gathered 17 years of observation data on foraging flocks and tracked 13 complete families with GPS transmitters. We explored how social status and family size affected wintering site choice and migration timing as well as how and why family sizes decreased. We found that family size decreased strongly during autumn migration, likely from juvenile death due to insufficient fuelling. It further decreased through the winter, here seemingly by juveniles accidentally splitting off during strong disturbance events. Different from previous findings, a large proportion of juveniles became independent during winter. Large families generally arrived later to the wintering grounds, wintered further from the breeding grounds and departed later for spring migration than adults without young. Independent young left for spring migration last. Thus, White-fronted Geese are differential migrants by social status. In combination with the observation of low breeding success in this population in recent years, our findings improve the understanding of its spatial and temporal patterns during winter, and their apparent changes. This can support conservation and management decisions for both White-fronted Geese as well as other large migrants with complex age and social structure.
Zusammenfassung
Dynamik der Familiengröße bei überwinternden Gänsen
Die Populationen der meisten Vogelarten setzen sich aus Individuen verschiedener Größe und sozialem Status zusammen. Gänsefamilien und -schwärme gehören dabei zu den besser bekannten. Wie jedoch der Verband von juvenilen Gänsen mit ihren Eltern als Familie Zugtiming und Raumnutzung der Population bestimmen und welche Ereignisse zur Unabhängigkeit von Juvenilen führen, ist bisher nicht ganzheitlich verstanden. Wir haben hier untersucht, wie Familiengrößen von Blässgänsen Anser a. albifrons sich in ihren Überwinterungsgebieten in den Niederlanden und Norddeutschland über den Winter entwickeln. Dazu haben wir 17 Jahre Beobachtungsdaten fressender Gänsegruppen gesammelt und 13 vollständige Familien mit GPS-Sendern verfolgt. Es wurde untersucht, wie sozialer Status und Familiengröße sich auf die Wahl des Überwinterungsgebietes und Zugtiming auswirken, und wie und warum Familien sich trennen. Wir konnten zeigen, dass Familiengrößen während des Herbstzuges stark abnehmen, vermutlich durch Tod der Jungtiere aufgrund ungenügender Fettreserven. Sie nahm durch den Winter weiter ab, hier möglicherweise durch unabsichtliche Trennung während starker Störungsereignisse. Entgegen früherer Erkenntnisse, ist ein Großteil der Juvenilen im Winter auf diese Weise unabhängig geworden. Große Familien sind im Allgemeinen später in den Überwinterungsgebieten angekommen, haben weiter von den Brutgebieten überwintert und sind im Frühjahr später abgezogen als Adultvögel ohne Jungtiere. Unabhängige Juvenile haben den Frühjahreszug als letzte begonnen. Folglich sind Blässgänse differentielle Migranten bedingt durch verschiedenen sozialen Status. In Kombination mit der Beobachtung, dass diese Population seit einigen Jahren sehr geringen Bruterfolg hat, verbessern unsere Ergebnisse das Verständnis der räumlichen und zeitlichen Populationsmuster im Wintergebiet und ihre Änderungen. Dies kann Artenschutz- und Managemententscheidungen für Blässgänse und auch andere große Migranten mit komplexer Alters- und sozialen Struktur unterstützen.
Similar content being viewed by others
Introduction
Many animals live in groups, and animal groups often show age and social structure. Two common examples of social groups are adult individuals bonded to form a breeding pair and pairs, or occasionally single adults along with their offspring, which form a family (Krause 2002). Animals living in families gain benefits from group-living such as decreased predation risk, while costs such as increased competition are shared with related individuals and offset by increased inclusive fitness (Hamilton 1964). Many species also spend part of the year in larger aggregations when family groups and independent individuals gather at resource sites (Krause 2002). The often enormous foraging and migratory flocks of large waterfowl such as geese Anserini, which are associated in families over the breeding season, are among the best known examples of non-breeding aggregations. Large flocks allow individuals to access enhanced group-living benefits such as increased anti-predator vigilance and information transfer (Roberts 1996; Krause 2002). Maintaining family bonds in flocks is beneficial as larger social units have been found to be dominant over small ones (Lamprecht 1986; Gregoire and Ankney 1990; Poisbleau et al. 2006). This allows large families to occupy optimal foraging positions on often crowded foraging sites in the winter (Black and Owen 1989; Black et al. 1992).
Juvenile mortality between hatching and arrival on the wintering grounds leads to the observation of reduced family sizes when migrating goose flocks arrive at wintering sites; both as a result of migration-related mortality and density dependent effects on the breeding grounds prior to onset of migration (Black and Owen 1989; Francis et al. 1992; Menu et al. 2005). Of the families that arrive on the wintering grounds with their juveniles, smaller-bodied goose species in large flocks seem to dissolve most families in winter (Johnson and Raveling 1988; Jónsson and Afton 2008), whereas larger goose species maintain family bonds through one or more winters (Ely 1979; Warren et al. 1993; Kruckenberg 2005). This suggests that a combination of juvenile condition, competition, and dominance in flocks may influence the persistence of goose family bonds (Johnson and Raveling 1988; Jónsson and Afton 2008). Family size decrease and family dissolution, i.e., the loss of some or all juveniles, have been linked to the onset of spring (Prevett and MacInnes 1980; Johnson and Raveling 1988; Black and Owen 1989), increased levels of parental aggression toward juveniles (Black and Owen 1989; Poisbleau et al. 2008; Scheiber et al. 2013) and the higher exposure of families to hunting (Madsen et al. 2002; Madsen 2010; Guillemain et al. 2013; Clausen et al. 2017). Still, accidental events, such as the separation of geese from their families during chaotic flock take-offs cannot be ruled out (Prevett and MacInnes 1980). Juvenile geese are also less adept flyers than adults (Green and Alerstam 2000) and may not be able to keep up with their parents, leading to separation (Black and Owen 1989; Francis et al. 1992). Such differences in movement capacity and dominance between social classes can lead to differential migration and winter distributions (see Cooke et al. 1975; Cristol et al. 1999). For example, larger, more dominant goose families have been hypothesised to displace smaller social units to sub-optimal southern wintering sites, leading to a latitudinal gradient with more juveniles in northern sites closer to the Arctic breeding grounds (Schamber et al. 2007). In addition to this social dominance effect (Gauthreaux 1982), the distribution of wintering geese is dependent on weather; geese avoid conditions that impede foraging such as deep snow and ice (Philippona 1966; Lok et al. 1992).
Greater White-fronted Geese Anser a. albifrons, hereafter White–fronted Geese, of the Baltic-North Sea flyway population have distinct family structure and aggregate in wintering flocks across large parts Western Europe (Madsen et al. 1999). It is also a species that is hunted or subject to derogation shooting during autumn migration and during winter in nearly all countries of its wintering range (except Belgium and the UK; Stroud et al. 2017). Hence, they provide an interesting opportunity to investigate the development of wintering goose family sizes just after autumn migration and the consequent spatio-temporal distributions of different population classes during winter.
In this paper we test a number of hypotheses related to migration and wintering strategies of goose families and the way family bonds persist: (1) Family size is reduced after autumn migration (2) Family size affects wintering site distance from the breeding grounds (3) Larger families winter in small flocks (4) Family size decreases over the winter (5) Family separation events are related to flight frequency. For this purpose, we draw on one of the few sets of family size counts from the Russian breeding grounds, long-term age ratio counts in wintering flocks in the core wintering range and high frequency GPS tracks of families of White-fronted Geese from their wintering grounds in the Netherlands and northern Germany.
Methods
Age ratios and family size
To estimate the effect of autumn migration on family size, we determined family sizes on the summer breeding grounds on Kolguev Island, Russia (approx. 69°N, 49°E, Fig. 1b) in August 2016, approximately one month prior to the onset of autumn migration. We directly observed groups of geese, counted the number of individuals, and identified adults and juveniles on the basis of evident differences in size and plumage. We assessed family size, i.e., the number of juveniles associated with one or both parents, relying on characteristic behaviour and social interactions between the juveniles and adults. A sample of 110 such observations of families and pairs without juveniles were collected, forming dataset A (Table 1), with a mean family size of 2.26 (range 0–6) when including the few families without young, and 2.38 (range 1–6) excluding pairs without young. As the two values do not differ much, and pairs without young have not been systematically assessed during winter, we used only the latter for further analyses. Kolguev Island hosts a third of the Western Palearctic population of White-fronted Geese, and we consider it as a proxy for the geographical situation of the breeding grounds as a whole (Kondratyev and Zaynagutdinova 2008; Kruckenberg et al. 2008), especially when calculating distances between breeding and wintering areas.

(adapted from Madsen et al. 1999)
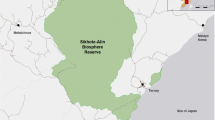
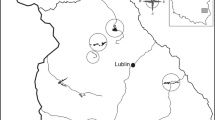
a Wintering grounds of White-fronted Geese in the Netherlands and northern Germany, showing 123 sites (filled and open circles) where the age ratio of 7149 flocks was determined (dataset B), and the subset of 65 sites (filled black circles) where size of 51,037 successful families were recorded in 1884 flocks (dataset C). Shaded grey area bounds 10,635 neckband-resightings (dataset D). Also, 21 split events (diamonds) were observed in 13 GPS tracked families (from dataset E). Observations correspond well with major rivers (marked as black lines). b Breeding grounds (ellipse) in Russia with Kolguev Island and general flyway (arrow) to wintering area (rectangle)
In autumn and winter, approx. 70 volunteer ornithologists observed 7149 foraging flocks of wintering White-fronted Geese at 123 sites across the study area in the Netherlands and northern Germany (see Fig. 1a), constituting the core wintering area of the species (Madsen et al. 1999). Field observations were carried out from goose arrival in September until goose departure in April, in 2000–2017. Most observations were made from October through January (81%), gradually declining through February (11%) and March (7%). September and April had far fewer observations, as most geese had not then arrived, or had already departed, respectively. The number of adult and juvenile birds in each flock, or a sample within especially large flocks was counted, using plumage characteristics and moult patterns throughout the wintering season (Koffijberg 2006). We calculated the flock’s age ratio as the proportion of juveniles within the sampled part of the flock. For all observations, we also counted the total number of individuals within each flock (i.e., total flock size), leading to a difference between total flock size and sampled size; the latter was used to determine age ratio. This formed dataset B (Table 1). Total flock size was 712 individual geese on average (range 2–20,000, 95th percentile P95 = 2500), and had a mean age ratio of 0.18 (range 0–0.87, P95 = 0.35). In a subsample of 1884 flocks at a subset of 65 sites, a specific group of 17 expert observers assessed goose family size using the same method and definitions as on Kolguev Island (see above). As a result, 51,037 families were counted, and formed dataset C (Table 1). The mean family size in wintering flocks was 1.78 juveniles (range 1–10).
To strengthen our conclusions, we augmented our data by including information on family size from observations of wintering neck-banded White-fronted Geese submitted by citizen scientists to the https://www.geese.org portal during the study period. As this additional social information is not compulsory when submitting sightings of ringed geese, the dataset is much smaller compared to age ratio counts in the field, but it is mainly provided by observers with expert knowledge. This approach has previously yielded robust results when used to supplement data on goose distributions and breeding status in the wintering area (see Clausen et al. 2018). We took additional steps to ensure that the data we used in analyses were reliable; these are detailed in the supplementary material (Online resource 01), and rejected 41% of the original ~ 18,000 observations gathered. In the remaining data, 626 sightings on average were reported each year (range 62–1143). We assessed family size after filtering out observations of single birds without young, and birds known to be under two years of age, thus not actively taking part in the breeding population. There were 10,635 observations of 4063 unique geese from 8416 sites obtained, forming dataset D (Table 1, Fig. 1a). In contrast with datasets B and C, this included observations of pairs without associated juveniles, i.e., a family size of 0. Datasets B and C included details on the observer and habitat type, while dataset D did not; dataset D also did not hold information on total flock size. Each bird in dataset D was seen 2.62 times on average over the study period (range 1–78), and had a family size of 0.59 (range 0–11). Approximately 71% of observations reported no juveniles associated with the banded bird; indeed, 59% of banded individuals were never observed with juveniles over the entire study period.
Family tracking
We caught and fitted 13 complete goose families (26 adults, 38 juveniles) with GPS transmitters in the Netherlands between November and January in 2013/14 (n = 3 families), 2014/15 (n = 4), and 2016/17 (n = 6). Large families were preferentially selected for tagging. In 2013/14 and 2014/15 we used e-obs GmbH backpacks with teflon harness (weight 45 g), and in 2016/17 madebytheo neck-band integrated devices (weight 35 g). Transmitters reported positions at 30-min intervals. Families were tracked within the study area (2–10°E, 50–54°N) during autumn and winter (tagging date—1 April) for 34–135 days.
We identified the day and position where a decrease in the number of family members within 1000 m of a reference bird relative to the previous day was recorded (Fig. 1a), and termed this a split. As reference bird for each family, we chose the parent with the greater number of GPS fixes. We determined the daily split probability by a binomial fit on the classification of each day as a success or failure (1 or 0) depending on whether a split occurred or not. To relate the probability of a split to goose flight, we summed the distance travelled by each family each day, and identified and counted the number of displacements with a speed ≥ 1000 m/30 min. This constituted dataset E (Table 1). Families travelled on average 11 km each day over the tracking period (range 0–306 km). On average, they travelled a distance ≥ 1000 m in 30 min twice each day (range 0–10). There were 21 split events recorded, of which 19 were juveniles separating from the family.
Context data
In order to analyse changes in age ratios and family sizes relative to the annually varying date of goose arrival on and departure from the wintering grounds, we extracted the first and last 90th percentile peaks in each year from 6266 daily observations of visible overhead migration in the Netherlands (https://www.trektellen.nl; Van Turnhout et al. 2009) as a proxy for annual arrival and departure dates. Data were pooled over 84 spring and 180 autumn counting sites. We excluded observations from sites close to night roosts, and observations which did not match the direction of migration appropriate to the season in order to avoid bias by local movements. Geese arrived between 26 September and 30 October, and departed between 3 March and 1 April, resulting in an average wintering period of 165 days (range 124–183). Following Madsen et al. (2002), we refer to the period 0–90 days after arrival in each year (ending approximately 31 December) as the autumn season, and the period 91–180 days (ending approximately 31 March) as the winter season.
To test whether spatial patterns in age ratios and family size could be explained by environmental conditions, we gathered the daily minimum temperature from 51 meteorological stations in the Netherlands and northern Germany (Koninklijk Nederlands Meteorologisch Instituut, Netherlands, n = 50; Deutscher Wetterdienst, Germany, n = 1). Meteorological stations were on average 19 km (range 4–61 km) from the positions at which flocks (datasets B, C) were observed, and 21 km (range 0.42–172 km) from locations at which neck-banded individuals (dataset D) were resighted.
Analyses
We first tested (1) whether family size was different on the breeding grounds of Kolguev Island one month prior to migration, and on the wintering grounds up to two months following the first arrivals of geese (hypothesis 1; dataset A). We then ran models to test (2) whether the distance from the breeding grounds (distance from Kolguev Island (49°E, 69°N); see Methods: Age ratios and family size) at which a family was observed was explained by the family’s size and the days since autumn arrivals (hypothesis 2; dataset C, D), and whether (3) family size was explained by flock size and the number of days since autumn arrival (hypotheses 3, 4; dataset C, D). Finally, we tested (4) whether the daily probability of a family splitting was explained by the days since goose arrivals, the daily distance travelled, the daily number of flights, and the daily family size (hypothesis 5; dataset E). See Table 2 for model details, including sample sizes.
In order to seek explanatory variables for our observations, we examined (5) how flock size was related to days since autumn arrival, temperature and distance from the breeding grounds. Further, using dataset B, we explored (6) flock age ratio (7) proportion of independent juveniles in a flock (called ‘juvenile independence ratio’) and (8) the proportion of family-associated birds in relation to flock size, days since autumn arrival, temperature and distance from the breeding grounds. The juvenile independence ratio was calculated as one minus the sum of all family sizes in a flock (family-associated juveniles) divided by the total number of juveniles in the flock: \( 1 - \frac{{\mathop \sum \nolimits_{{x = 1}}^{{x = n}} {\text{family}}\,{\text{size}}_{i} }}{{{\text{total}}\,{\text{number}}\,{\text{of}}\,{\text{juveniles}}}}.\)
We calculated the different, but related proportion of family-associated birds as one minus the sum of all family sizes plus twice the number of families divided by the total flock size (see Table 2):
We used a one-way Kruskal–Wallis test with a Nemenyi post-hoc for model 1, Poisson generalised linear mixed models (GLMMs) for models 2, 3, and 4, and binomial-error GLMMs for models 5, 6, 7, and 8. In each model, we included the breeding year, the observer identity, and the goose identity as independent random effects, if available in the relevant data (see Table 2). Analyses and data handling were performed in the R environment (R Core Team 2017), using the packages lme4 (Bates et al. 2015), mgcv (Wood 2013) and move (Kranstauber et al. 2018).
Results
Autumn migration and family size
Family sizes observed on the breeding grounds on Kolguev Island (dataset A, mean ± 95% confidence interval = 2.38 ± 0.25 juveniles) were significantly higher than family sizes from flock counts (dataset C, mean ± 95% confidence interval = 1.72 ± 0.0398) and neck-banded birds (dataset D, mean ± 95% confidence interval = 0.46 ± 0.124) recorded in the first two months following initial goose arrivals on the wintering grounds. This indicates an average loss of approximately 0.5 (dataset C) and 1.8 juveniles (dataset D), respectively, along the migration route (model 1: Kruskal–Wallis rank sum test, χ2 = 469.11, P < 0.001; see Fig. 2). All pairwise differences were significant (Nemenyi post-hoc tests, D-B: χ2 = 17.515, P < 0.001; D-C: χ2 = 248.536, P < 0.001; B-C: χ2 = 436.709, P < 0.001).
Mean family size ± 95% confidence interval (vertical lineranges) in the core breeding grounds on Kolguev Island in August 2016 (dataset A), one month prior to migration (August 2016), and in the core wintering range in the Netherlands and northern Germany from two different datasets (counting data = dataset C, marked birds = dataset D), observed within the first 60 days of goose autumn arrivals in 2016. Note that values for both dataset C (without zero family sizes) and dataset D (with zero family sizes) can be compared with the Kolguev Island family size, as the latter is basically the same value if including or excluding size zero families. For dataset C the 95% CI 0.02, and is too small to be visible
Family size and wintering site
Family size alone was not a good predictor of wintering site distance from the breeding area in dataset C (model 2: z = 1.806, P = 0.071), and in dataset D (model 2: z = 0.516, P = 0.606). The season (i.e., autumn or winter) alone did not appear to be a significant predictor of wintering distance in dataset C (model 2: z = − 0.683, P = 0.495), but was a significant effect in dataset D (model 2: z = 3.391, P < 0.01). However, the minimum daily temperature explained observed variation well in both dataset C (model 2: z = 3.698, P < 0.01), and dataset D (model 2: z = − 9.346, P < 0.01), though the trends ran counter to each other. Interestingly, the interaction term of family size and season was found to influence wintering site distance only in winter, but not in autumn in dataset C (model 2: z = 4.009, P < 0.01; Fig. 3a), and not at all in dataset D (model 2: z = − 1.851, P = 0.064; Fig. 3b). Thus, on average in dataset C, a family with two juveniles would be found 4 km further from the breeding grounds in winter as opposed to autumn, while a large family of seven juveniles would be 26 km further away.
GLMM fits (lines) and mean distance of wintering sites from Kolguev Island (symbols) per family size for a family size counts (dataset C) and b neckband-resightings (dataset D). Data and fits are separated by number of days since arrival to the wintering grounds: 0–90 days (solid lines, circles), 91–180 days (dashed line, squares). Vertical lineranges represent 95% confidence intervals at each data point
Flock size, days since arrival and family size
Total flock size and days since arrival were good predictors of family size and both had a negative effect in dataset C (model 3: flock size z = − 4.288, P < 0.001; days since arrival z = − 8.669, P < 0.001; see Fig. 4a). On average, families in this dataset had two juveniles in the first 10 days after arrival, which decreased linearly to 1.49 juveniles per family 180 days after arrival, i.e., just prior to departure. Similarly, families in flocks of up to 100 geese had 1.91 juveniles, but only 1.67 juveniles in large flocks of around 4000 geese. Family size in dataset D was negatively affected by days since arrival (model 3: z = −5.018, P < 0.001; Fig. 4a) with families of a mean 0.593 juveniles in the first ten days after arrival, and 0.497 juveniles 180 days after arrival.
a Mean family size in 10 day intervals since goose autumn arrival (symbols) in flock counts (dataset C, circles), neckband-resightings (dataset D, squares) and GLMM fits (lines) for model 3. Triangles and the dot-dash line represent the mean proportion of first winter juveniles observed in flock counts (dataset C), which are not associated with parents and the GLMM fit for model 7. Vertical lineranges and shaded grey areas represent 95% confidence intervals at each point. b Family size development over winter (circles) in 9 of 13 GPS tracked families (family identity in header bar). The first circle in each sub-plot indicates family size immediately after the start of tagging, while the last circle represents family size at the end of the tracking period (either 1 April or when the family crossed the 8°E longitude line). Symbols overlap when events are temporally proximate
Flight frequency, distance, and split probability
We found a strong correlation between days since arrival and family size in dataset E (Pearson’s r = 0.728; Fig. 4b). To avoid biasing our model by this multi-collinearity, we chose to omit days since arrival in the final model, reasoning that we had adequately characterised the development of family sizes over the course of winter. The daily split probability of families was significantly lower in larger families (model 4: z = − 2.644 P = 0.0082); for example, the smallest families with one juvenile (Psplit = 0.026) were on average five times more likely to split than a family with five juveniles (Psplit = 0.00542; Fig. 5a). While the number of flights per day seemed to have no effect (z = − 0.355, P = 0.723), the distance travelled per day increased the likelihood of splitting (z = 2.12, P = 0.027); a family covering up to 80 km/day had a split probability (Psplit = 0.092) around 5.6 times that of a family travelling only 10 km/day (Psplit = 0.016; Fig. 5b).
Spatio-temporal trends in flocks
Our auxiliary analyses showed that goose flocks were larger at wintering sites which were closer to the breeding grounds (model 5: z = − 100.61, P < 0.001), larger later in the wintering period (z = 77.20, P < 0.001) and also larger at lower minimum temperatures (z = − 31.32, P < 0.001). Thus, the average flock at the eastern margins of the study area, 2760–2790 km from breeding sites, comprised 543 individuals and was 1.45 times larger than flocks furthest away (3040–3070 km) with 374 geese. Furthermore, the average late winter flock (170–180 days after arrivals) held 458 geese and was, thus, 1.22 times the size of the average early autumn flock (0–10 days) of 376 geese. Flocks had on average 582 geese on days when the minimum temperature was between – 1 and 1 °C, growing to 680 birds when temperatures were – 13 to − 11 °C.
The overall age ratio of flocks was not affected by the distance of the wintering site from the breeding grounds (model 6: z = − 1.3460.965, P = 0.178335). However, it was significantly higher later in the wintering period (z = 1.2073.218, P = 0.2270013); for example, flocks seen between 0 and 10 days after arrival had an average age ratio of 0.157 juveniles in the flock, while flocks in late winter (170–180 days) had a mean age ratio of 0.256 (see Fig. 6d). Larger flocks also had a significantly lower age ratio, from 0.224 in flocks of size 0–100 geese, to 0.135 in extremely large flocks of around 3000 geese (z = − 4.227, P < 0.001).
Mean values (symbols) and GLMM fits (lines) for a the proportion of flocks composed of family-associated geese at different flock sizes, and b the proportion of family-associated birds in a flock as a function of the days since autumn arrivals c the proportion of flock juveniles not associated in a family as a function of flock size and d the overall juvenile proportion of flocks as a function of days since autumn arrivals. Vertical lineranges and shaded grey areas show 95% confidence intervals in all cases
The proportion of geese associated in families was lower in larger flocks: flocks of approximately 3000 geese had a mean family-associated proportion of only 0.102, while flocks half the size (in the range of 1400–1500) and much smaller flocks (0–100) had family-associated proportions of 0.163 and 0.469, respectively (model 8: z = 13.117, P < 0.001; Fig. 6a). This proportion increased through the winter, such that on average a flock just after the beginning of arrivals (0–10 days) had a family-associated proportion of 0.264, whereas the same for a flock in late winter (170–180 days) was 0.367 (z = 2.124, P = 0.034; Fig. 6b). However, the proportion was insensitive to the distance from the breeding grounds at which flocks were seen (z = − 0.036, P = 0.9713).
The proportion of independent juveniles increased with flock size, such that the average small flock (0–100 geese) had a juvenile independence ratio of 0.037, while flocks of intermediate (1400–1500 geese) and large sizes (around 3000 geese) had ratios of 0.143 and 0.232, respectively (model 7: z = − 8.063, P < 0.001; Fig. 6c). This proportion rose through winter, with early autumn just after goose arrivals (0–10 days; mean value = 0.034) contrasting strongly with late winter (160–170 days; mean value = 0.273; z = − 4.315, P < 0.001; Fig. 4a). We found that the proportion of independent juveniles was not affected by the distance of the wintering site from the breeding grounds (z = − 1.267, P ≤ 0.001).
Discussion
We determined family sizes and age ratios of White-fronted Geese in late summer on the breeding grounds and after autumn migration in their wintering areas. We evaluated these data regarding different scales of social organisation of goose families and flocks to improve our understanding of spatio-temporal patterns in the context of migration and winter conditions. In support of our hypotheses, we found that autumn migration takes a significant toll on goose families, family sizes dropping significantly when arriving on the wintering grounds. On the wintering grounds then, families leverage their social dominance to occupy climatically milder sites further from the breeding grounds in winter, where goose flocks are also smaller. Over the winter, families become smaller, but large and more sedentary families are less likely to split or lose members to predation or hunting. Family split events were shown to relate to flight intensity, thus likely being accidental due to disturbance events. The age ratio of flocks increased significantly through the winter, with a marked increase in the proportion of independent juveniles as well as the proportion of family-associated birds just before spring migration.
Autumn migration and family size
Our result that families were significantly larger on the breeding sites at Kolguev Island shortly prior to migration than in the first two months on the wintering grounds confirms our first hypothesis, and is in line with previous findings of high autumn migration mortality in juvenile Arctic geese (Owen and Black 1989; Francis et al. 1992; Menu et al. 2005). These studies concluded that autumn migration-related mortality in Arctic goose populations is mainly due to strong density-driven competition for resources prior to and during autumn migration, which leads to juveniles being under-fueled for the journey and thus having a lower survival rate. Autumn migration in our population of White-fronted Geese has one important stopover site for fuelling close to the breeding grounds, but is then characterised by a non-stop, quick flight to the wintering grounds (Kölzsch et al. 2016). Such a long-distance flight might be problematic for juveniles if not properly fueled.
Juvenile summertime survival of Arctic-breeding birds including geese has earlier been strongly linked to the cyclical abundance of and concomitant levels of predation on Arctic rodents Arvicolinae in the ‘alternative prey hypothesis’ (Summers and Underhill 1987). However, Kolguev Island lacks lemmings and their associated cycle (Kondratyev and Zaynagutdinova 2008; Kruckenberg et al. 2008), and young geese form the regular, main prey of most predators there (Pokrovsky et al. 2015). This is shown to result in strong family size decreases between hatching (early July; mean ≈ 3) and fledging (end July; mean ≈ 2; see Kondratyev and Zaynagutdinova 2008). We report nearly the same mean family size pre-migration (mid-August; mean = 2.38) as at fledging, suggesting both that we sampled in a year with typical predation levels, and that most juveniles in August were sufficiently grown to avoid all but the largest predators (such as Arctic Fox Vulpes lagopus, Rough-legged Buzzard Buteo lagopus and Glaucous Gull Larus hyperboreus). Finally, adult geese are effective at countering predation attempts on their young (Thompson and Raveling 1987), allowing the conclusion that reductions in family size likely occurred during autumn migration, rather than between our sampling and the start of migration.
Temporal dynamics of family size
Family size continues to decrease after arrival on the wintering grounds pointing to a steady reduction in the number of juveniles associated in families, as expected in hypothesis 4. There is, however, high variation in the time of juvenile independence; some young geese may be found with parents even in their second or third winter (Ely 1979; Warren et al. 1993; Kruckenberg 2005). Hunting takes a toll on geese and on waterfowl more generally, and there is good evidence from both our study area and across Europe that juveniles and families are over-represented in hunting bags (Madsen 2010; Guillemain et al. 2013; Clausen et al. 2017). While this may present a simple explanation for observed family size reductions, the simultaneously increasing proportion of independent juveniles in flocks suggests that though hunting may be a leading cause of juvenile autumn mortality (most hunting seasons end around January), young geese also become independent rather than only being taken out from the population. This is in line with the finding that juvenile mortality is unrelated to hunting in the Pink-footed Goose A. brachyrhynchus which also winters in parts of the study area (Madsen et al. 2002).
Juvenile independence from parents should result in a consequently steady reduction in the proportion of family-associated birds in flocks over the winter, but our findings showed the opposite. We suggest the possible explanation that families with juveniles may begin spring migration later than pairs without young, mirroring their differential autumn migration arrival, which is later than that of single and paired geese (Jongejans et al. 2015). This proposed differential migration is further supported by the strong temporal increases in proportions of juveniles in foraging flocks. We hypothesise that in spring, single and paired geese without young leave the wintering grounds for Arctic breeding sites first, followed by families with young and finally independent juveniles. While this is contrary to previous work on the spring migration of Pink-footed Geese (Madsen 2001), it is interestingly similar to the migration of some juvenile Snow Geese, which, having separated from their parents, appear to arrive somewhat later to the breeding grounds than adults (Prevett and MacInnes 1980).
Data from our GPS-tracked goose families support our fifth hypothesis that winter dissociation of juveniles from their families is accidental (Prevett and MacInnes 1980), taking place when geese make long-distance movements, but not necessarily when the number of flights is high. This may indicate that only disturbances sufficient to compel geese to relocate to distant sites prompt family splitting. In most cases, juveniles split off one at a time and not all at once. In the chaotic take-off conditions hypothesised to promote accidental separation of individuals from their families (Prevett and MacInnes 1980) larger families might be easier to adhere to, possibly explaining why they are less likely to split.
Spatial dynamics of family size
Our results show that flock sizes were smaller further from the breeding grounds, which can be explained by the metabolic constraints that determine maximum flight distance and duration of migration (Klaassen 1996). GPS tracks of geese from Kolguev Island in the autumns of 2014–2016 showed that many geese used short stopovers in Poland or northern Germany before entering our study area (unpublished data, see also (Kölzsch et al. 2016). Thus, flight restrictions might already have been active earlier, especially for geese travelling with young. This is supported by the fact that large goose families had moved further away from the breeding sites (i.e., further into the study site) only in the winter season (90–180 days after arrival), after having refuelled in more easterly sites.
Once all geese had arrived and foraging sites became saturated, food seems to have been more limited and was eventually also limited by snow cover. It seems that large, socially dominant families then moved further to occupy climatically milder sites which are more favourable (Vangilder and Smith 1985, Schamber et al. 2007). This supports both our second hypothesis and the earlier suggestion of differential use of wintering sites according to social status (Jongejans et al. 2015) and is in line with current knowledge of goose foraging preferences (Fox and Madsen 2017). Geese tolerate snow depths of about 15 cm before relocating to areas with better access to forage (Philippona 1966). Such conditions have become rare in the study area over the past decade, and usually do not occur before winter. This might explain why spatial differences in family size are only observed in winter and not autumn. Limited habitat availability in the western Netherlands and Belgium, combined with high hunting pressure in France and the high energetic costs of long flights, seems to lead to geese aggregating in large flocks on the remaining accessible grassland sites on cold, snowy days.
Finally, our results indicate that larger families were associated with smaller flocks, confirming our third hypothesis. This finding in combination with the spatial pattern of large families wintering further from the breeding grounds might allow the conclusion that larger flocks have a lower proportion of juveniles overall (Jongejans et al. 2015). However, large flocks had a higher proportion of independent juveniles and a correspondingly lower proportion of geese associated in families, suggesting that, as juveniles separate from their parents, they gather with other juveniles and pairs of adults in flocks to gain group-living benefits (Prevett and MacInnes 1980). However, contrary to expectations that both the above proportions would be well predicted by distance to the breeding grounds, no such trends were found. This could be linked to population movements in late winter and early spring, when geese seem to spread out across the study area to prepare for the spring migration (Kölzsch et al. 2016).
Conclusions
Overall, we show that in addition to well-known ecological factors like food availability, disturbances or site faithfulness, social status also influences large-scale goose movements. Migration has a strong negative effect on goose family size, families can leverage social status (related to family size) when selecting wintering sites despite arriving later and the spring migration timing of families is likely delayed relative to pairs without juveniles. Furthermore and in contrast with earlier studies (Kruckenberg 2005), our data suggest that large numbers of White-fronted Goose juveniles reach independence each winter; juveniles who cannot keep up with their families when travelling over large distances may contribute to this trend.
These results have implications for conservation; a disproportionately high number of productive adults (in families) is present on the wintering grounds in early spring, where they are susceptible to management actions such as hunting or derogation shooting (Jongejans et al. 2015). Further, juvenile survival is a major component of population growth rate and the presence (and susceptibility) of a similarly disproportionate number of juveniles makes the late wintering period critical to management. It is thus imperative to closely monitor population development (Stroud et al. 2017; Lefebvre et al. 2017) and take into account social structure driven population dynamics as we present here.
References
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Black JM, Owen M (1989) Agonistic behaviour in barnacle goose flocks: assessment, investment and reproductive success. Anim Behav 37 Part 2:199–209. https://doi.org/10.1016/0003-3472(89)90110-3
Black JM, Carbone C, Wells RL, Owen M (1992) Foraging dynamics in goose flocks: the cost of living on the edge. Anim Behav 44:41–50
Clausen KK, Christensen TK, Gundersen OM, Madsen J (2017) Impact of hunting along the migration corridor of pink-footed geese Anser brachyrhynchus—implications for sustainable harvest management. J Appl Ecol 54:1563–1570. https://doi.org/10.1111/1365-2664.12850
Clausen KK, Madsen J, Cottaar F et al (2018) Highly dynamic wintering strategies in migratory geese: Coping with environmental change. Glob Change Biol 24:3214–3225. https://doi.org/10.1111/gcb.14061
Cooke F, MacInnes C, Prevett J (1975) Gene flow between breeding populations of Lesser Snow Geese. Auk 92:493–510
Cristol DA, Baker MB, Carbone C (1999) Differential migration revisited. In: Nolan V Jr, Ketterson ED, Thompson CF (eds) Current ornithology. Springer, New York, pp 33–88
Ely CR (1979) Breeding biology of the white-fronted goose (Anser albifrons frontalis) on the Yukon-Kuskokwim Delta, Alaska. M.S., University of California, Davis
Fox AD, Madsen J (2017) Threatened species to super-abundance: the unexpected international implications of successful goose conservation. Ambio 46:179–187. https://doi.org/10.1007/s13280-016-0878-2
Francis CM, Richards MH, Cooke F, Rockwell RF (1992) Long-term changes in survival rates of Lesser Snow Geese. Ecology 73:1346–1362. https://doi.org/10.2307/1940681
Gauthreaux SA Jr (1982) The ecology and evolution of avian migration systems. In: Farner DS, King JR, Parkes KC (eds) Avian biology. Academic Press, Amsterdam, pp 93–168
Green M, Alerstam T (2000) Flight speeds and climb rates of Brent Geese: mass-dependent differences between spring and autumn migration. J Avian Biol 31:215–225. https://doi.org/10.1034/j.1600-048X.2000.310213.x
Gregoire PE, Ankney CD (1990) Agonistic behavior and dominance relationships among lesser snow geese during winter and spring migration. Auk 107:550–560
Guillemain M, Fox AD, Pöysä H et al (2013) Autumn survival inferred from wing age ratios: Wigeon juvenile survival half that of adults at best? J Ornithol 154:351–358. https://doi.org/10.1007/s10336-012-0899-y
Hamilton WD (1964) The genetical evolution of social behaviour I. J Theor Biol 7:1–16. https://doi.org/10.1016/0022-5193(64)90038-4
Medina L, Schneider F (1988) Weak family associations in Cackling Geese during winter: effects of body size and food resources on goose social organization. Waterfowl Winter 71–89
Jongejans E, Nolet BA, Schekkerman H et al (2015) Naar een effectief en internationaal verantwoord beheer van de in Nederland overwinterende populatie Kolganzen. SOVON Vogelonderzoek, Nederland
Jónsson JE, Afton AD (2008) Lesser Snow Geese and Ross’s Geese form mixed flocks during winter but differ in family maintenance and social status. Wilson J Ornithol 120:725–731
Klaassen M (1996) Metabolic constraints on long-distance migration in birds. J Exp Biol 199:57–64
Koffijberg K (2006) Herkenning en ruipatronen van eerstejaars Kolganzen in de winter. Limosa 79:163
Kölzsch A, Müskens GJDM, Kruckenberg H et al (2016) Towards a new understanding of migration timing: slower spring than autumn migration in geese reflects different decision rules for stopover use and departure. Oikos 125:1496–1507. https://doi.org/10.1111/oik.03121
Kondratyev A, Zaynagutdinova E (2008) Greater White-fronted Geese (Anser albifrons) and Bean Geese (A. fabalis) on Kolguev Island—abundance, habitat distribution, and breeding biology. Vogelwelt 129:326–333
Kranstauber B, Smolla M, Scharf AK (2018) move: visualizing and analyzing animal track data. R package version 3.1.0. https://CRAN.R-project.org/package=move
Krause J, Ruxton GD (2002) Living in groups. Oxford University Press, Oxford
Kruckenberg H (2005) Wann werden `die Kleinen’ endlich erwachsen? Untersuchungen zum Familienzusammenhalt farbmarkierter Blessgänse Anser albifrons albifrons. Vogelwelt 126:253
Kruckenberg H, Kondratyev A, Mooij J et al (2008) White-fronted goose flyway population status. Angew Feldbiologie 2:77
Lamprecht J (1986) Structure and causation of the dominance hierarchy in a flock of Bar-Headed Geese (Anser Indicus). Behaviour 96:28–48. https://doi.org/10.1163/156853986X00207
Lefebvre J, Gauthier G, Giroux J-F et al (2017) The greater snow goose Anser caerulescens atlanticus: managing an overabundant population. Ambio 46:262–274. https://doi.org/10.1007/s13280-016-0887-1
Lok M, van den Berg L, Ebbinge B et al (1992) Numbers and distribution of wild geese in the Netherlands, 1984–89, with special reference to weather conditions. Wildfowl 43:107–116
Madsen J (2010) Age bias in the bag of pink-footed geese Anser brachyrhynchus: influence of flocking behaviour on vulnerability. Eur J Wildl Res 56:577–582. https://doi.org/10.1007/s10344-009-0349-1
Madsen J (2001) Spring migration strategies in Pink-footed Geese Anser brachyrhynchus and consequences for spring fattering and fecundity. Ardea 89:43–55
Madsen J, Cracknell G, Fox A (1999) Goose populations of the Western Palearctic. National Environmental Research Institute Denmark and Wetlands International, Wageningen
Madsen J, Frederiksen M, Ganter B (2002) Trends in annual and seasonal survival of Pink-footed Geese Anser brachyrhynchus: annual and seasonal survival of Pink-footed Geese. Ibis 144:218–226. https://doi.org/10.1046/j.1474-919X.2002.00045.x
Menu S, Gauthier G, Reed A, Holberton RL (2005) Survival of young greater snow geese (chen caerulescens atlantica) during fall migration. Auk 122:479–496. https://doi.org/10.1642/0004-8038(2005) 122[0479:SOYGSG]2.0.CO;2
Owen M, Black JM (1989) Factors affecting the survival of barnacle geese on migration from the breeding grounds. J Anim Ecol 58:603–617
Philippona J (1966) Geese in cold winter weather. Wildfowl 17:3
Poisbleau M, Desmonts D, Fritz H (2008) Dominance relationships in dark-bellied brent geese Branta bernicla bernicla at spring staging areas. Ardea 96:135–139
Poisbleau M, Fritz H, Valeix M et al (2006) Social dominance correlates and family status in wintering dark-bellied brent geese, Branta bernicla bernicla. Anim Behav 71:1351–1358. https://doi.org/10.1016/j.anbehav.2005.09.014
Pokrovsky I, Ehrich D, Ims RA et al (2015) Rough-legged buzzards, arctic foxes and red foxes in a tundra ecosystem without rodents. PLoS One 10:e0118740. https://doi.org/10.1371/journal.pone.0118740
Prevett JP, MacInnes CD (1980) Family and other social groups in Snow Geese. Wildl Monogr 3–46
R Core Team (2017) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna
Roberts G (1996) Why individual vigilance declines as group size increases. Anim Behav 51:1077–1086
Schamber JL, Sedinger JS, Ward DH, Hagmeier KR (2007) Latitudinal variation in population structure of wintering Pacific Black Brant. J Field Ornithol 78:74–82. https://doi.org/10.1111/j.1557-9263.2006.00087.x
Scheiber IB, Kotrschal K, Weiß BM, Hemetsberger J (2013) The social life of greylag geese. Cambridge University Press, Cambridge
Stroud DA, Madsen J, Fox AD (2017) Key actions towards the sustainable management of European geese. Ambio 46:328–338
Summers RW, Underhill LG (1987) Factors related to breeding production of Brent Geese Branta b. bernicla and waders Charadrii on the Taimyr Peninsula. Bird Study 34:161–171
Thompson SC, Raveling DG (1987) Incubation behavior of Emperor Geese compared with other Geese: interactions of predation, body size, and energetics. Auk 104:707–716
Van Turnhout C, Van Winden E, Troost G et al (2009) Veranderingen in timing van zichtbare najaarstrek over Nederland: een pleidooi voor hernieuwde standaardisatie van trektellingen. Limosa 82:68
Vangilder LD, Smith LM (1985) Differential distribution of wintering brant by necklace type. Auk 102:645–647
Warren SM, Fox AD, Walsh A, O’Sullivan P (1993) Extended parent-offspring relationships in greenland white-fronted Geese (Anser albifrons flavirostris). Auk 110:145–148
Wood SN (2013) Generalized additive models: an introduction with R, 2nd edn. Chapman and Hall/CRC, Baco Raton
Acknowledgements
Open access funding provided by Max Planck Society. We thank the many observers who participated in age ratio and family size counts of goose flocks, and those who determined social status when entering their sightings of marked geese on https://www.geese.org. Thanks to Yke van Randen who provided https://www.geese.org data, Gerard Troost who provided data from https://www.trektellen.org, the Dutch Association of Goose Catchers who caught goose families for tagging with GPS transmitters, and Helmut Kruckenberg and Peter Glazov who majorly contributed to the 2016 Kolguev expedition, which was funded by a National Geographic Society Grant to AK (GEFNE141-15). PRG was financially supported by the European Commission through the programme Erasmus Mundus Master Course - International Master in Applied Ecology (EMMC-IMAE) (FPA 532524-1-FR-2012-ERA MUNDUS-EMMC). We also thank one anonymous reviewer for helpful comments on an earlier version of this manuscript. This work complies with Dutch, German, and European law and follows applicable ethical standards. Approval for catching and tagging goose families was obtained from the Animal Welfare Committee of the Royal Netherlands Academy of Arts and Sciences (DEC NIOO13.14).
Funding
The 2016 Kolguev expedition was funded by a National Geographic Society Grant to AK (GEFNE141-15). PRG was financially supported by the European Commission through the programme Erasmus Mundus Master Course—International Master in Applied Ecology (EMMC-IMAE) (FPA 532524-1-FR-2012-ERA MUNDUS-EMMC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Approval for catching and tagging goose families was obtained from the Animal Welfare Committee of the Royal Netherlands Academy of Arts and Sciences (DEC NIOO13.14).
Additional information
Communicated by F. Bairlein.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Gupte, P.R., Koffijberg, K., Müskens, G.J.D.M. et al. Family size dynamics in wintering geese. J Ornithol 160, 363–375 (2019). https://doi.org/10.1007/s10336-018-1613-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-018-1613-5