IL-37 Expression Reduces Lean Body Mass in Mice by Reducing Food Intake
Abstract
:1. Introduction
2. Results
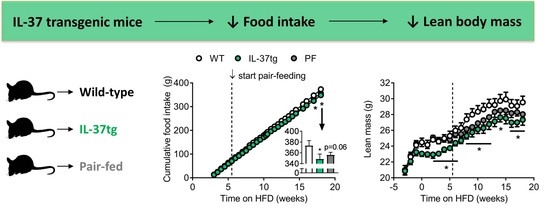
2.1. IL-37 Expression Alleviates Diet-Induced Weight Gain and Reduces Food Intake
2.2. Reduced Food Intake in Heterozygous IL-37tg Mice Decreases Lean Body Mass
2.3. IL-37 Expression Decreases Energy Expenditure in Conjunction with Lean Body Mass Reduction
3. Discussion
4. Materials and Methods
4.1. Animals and Diet
4.2. Body Weight, Body Composition and Food Intake
4.3. Plasma Glucose and Lipids
4.4. Energy Metabolism
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BP | Binding protein |
| HFD | High fat diet |
| IL | Interleukin |
| SLE | Systemic lupus erythematosus |
| tg | Transgenic |
| WAT | White adipose tissue |
| WT | Wild-type |
References
- Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; Naghavi, M.; et al. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [PubMed]
- Berrington de Gonzalez, A.; Hartge, P.; Cerhan, J.R.; Flint, A.J.; Hannan, L.; MacInnis, R.J.; Moore, S.C.; Tobias, G.S.; Anton-Culver, H.; Freeman, L.B.; et al. Body-mass index and mortality among 1.46 million white adults. N. Engl. J. Med. 2010, 363, 2211–2219. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.A. Diseases and disorders associated with excess body weight. Ann. Clin. Lab. Sci. 2011, 41, 107–121. [Google Scholar] [PubMed]
- Lumeng, C.N.; Saltiel, A.R. Inflammatory links between obesity and metabolic disease. J. Clin. Investig. 2011, 121, 2111–2117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borst, S.E. The role of tnf-alpha in insulin resistance. Endocrine 2004, 23, 177–182. [Google Scholar] [CrossRef]
- Van der Valk, F.M.; van Wijk, D.F.; Stroes, E.S. Novel anti-inflammatory strategies in atherosclerosis. Curr. Opin. Lipidol. 2012, 23, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Esser, N.; Paquot, N.; Scheen, A.J. Anti-inflammatory agents to treat or prevent type 2 diabetes, metabolic syndrome and cardiovascular disease. Expert Opin. Investig. Drugs 2015, 24, 283–307. [Google Scholar] [CrossRef] [PubMed]
- Moschen, A.R.; Molnar, C.; Enrich, B.; Geiger, S.; Ebenbichler, C.F.; Tilg, H. Adipose and liver expression of interleukin (IL)-1 family members in morbid obesity and effects of weight loss. Mol. Med. 2011, 17, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Nold, M.F.; Nold-Petry, C.A.; Zepp, J.A.; Palmer, B.E.; Bufler, P.; Dinarello, C.A. IL-37 is a fundamental inhibitor of innate immunity. Nat. Immunol. 2010, 11, 1014–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boraschi, D.; Lucchesi, D.; Hainzl, S.; Leitner, M.; Maier, E.; Mangelberger, D.; Oostingh, G.J.; Pfaller, T.; Pixner, C.; Posselt, G.; et al. IL-37: A new anti-inflammatory cytokine of the IL-1 family. Eur. Cytokine Netw. 2011, 22, 127–147. [Google Scholar] [PubMed]
- Rudloff, I.; Cho, S.X.; Lao, J.C.; Ngo, D.; McKenzie, M.; Nold-Petry, C.A.; Nold, M.F. Monocytes and dendritic cells are the primary sources of interleukin 37 in human immune cells. J. Leukoc. Biol. 2017, 101, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Imaeda, H.; Takahashi, K.; Fujimoto, T.; Kasumi, E.; Ban, H.; Bamba, S.; Sonoda, H.; Shimizu, T.; Fujiyama, Y.; Andoh, A. Epithelial expression of interleukin-37b in inflammatory bowel disease. Clin. Exp. Immunol. 2013, 172, 410–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, T.; Lin, Q.; Zhao, M.; Hu, Y.; Yu, Y.; Jin, J.; Zhou, H.; Hu, X.; Wei, R.; Zhang, X.; et al. Il-37 is a novel proangiogenic factor of developmental and pathological angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2638–2646. [Google Scholar] [CrossRef] [PubMed]
- Ballak, D.B.; van Diepen, J.A.; Moschen, A.R.; Jansen, H.J.; Hijmans, A.; Groenhof, G.J.; Leenders, F.; Bufler, P.; Boekschoten, M.V.; Muller, M.; et al. Il-37 protects against obesity-induced inflammation and insulin resistance. Nat. Commun. 2014, 5, 4711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bufler, P.; Gamboni-Robertson, F.; Azam, T.; Kim, S.H.; Dinarello, C.A. Interleukin-1 homologues IL-1f7b and IL-18 contain functional mrna instability elements within the coding region responsive to lipopolysaccharide. Biochem. J. 2004, 381, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kulk, N.; Nold, M.F.; Graf, R.; Kim, S.H.; Reinhardt, D.; Dinarello, C.A.; Bufler, P. The IL-1 family member 7b translocates to the nucleus and down-regulates proinflammatory cytokines. J. Immunol. 2008, 180, 5477–5482. [Google Scholar] [CrossRef] [PubMed]
- Bulau, A.M.; Nold, M.F.; Li, S.; Nold-Petry, C.A.; Fink, M.; Mansell, A.; Schwerd, T.; Hong, J.; Rubartelli, A.; Dinarello, C.A.; et al. Role of caspase-1 in nuclear translocation of il-37, release of the cytokine, and IL-37 inhibition of innate immune responses. Proc. Natl. Acad. Sci. USA 2014, 111, 2650–2655. [Google Scholar] [CrossRef] [PubMed]
- Coll-Miro, M.; Francos-Quijorna, I.; Santos-Nogueira, E.; Torres-Espin, A.; Bufler, P.; Dinarello, C.A.; Lopez-Vales, R. Beneficial effects of IL-37 after spinal cord injury in mice. Proc. Natl. Acad. Sci. USA 2016, 113, 1411–1416. [Google Scholar] [CrossRef] [PubMed]
- Bulau, A.M.; Fink, M.; Maucksch, C.; Kappler, R.; Mayr, D.; Wagner, K.; Bufler, P. In vivo expression of interleukin-37 reduces local and systemic inflammation in concanavalin A-induced hepatitis. ScientificWorldJournal 2011, 11, 2480–2490. [Google Scholar] [CrossRef] [PubMed]
- Van de Veerdonk, F.L.; Gresnigt, M.S.; Oosting, M.; van der Meer, J.W.; Joosten, L.A.; Netea, M.G.; Dinarello, C.A. Protective host defense against disseminated candidiasis is impaired in mice expressing human interleukin-37. Front. Microbiol. 2014, 5, 762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nold-Petry, C.A.; Lo, C.Y.; Rudloff, I.; Elgass, K.D.; Li, S.; Gantier, M.P.; Lotz-Havla, A.S.; Gersting, S.W.; Cho, S.X.; Lao, J.C.; et al. IL-37 requires the receptors IL-18Rα and IL-1R8 (SIGIRR) to carry out its multifaceted anti-inflammatory program upon innate signal transduction. Nat. Immunol. 2015, 16, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, G.; Justice, J.N.; Boyle, K.E.; D’Alessandro, A.; Eisenmesser, E.Z.; Herrera, J.J.; Hansen, K.C.; Nemkov, T.; Stienstra, R.; Garlanda, C.; et al. Interleukin 37 reverses the metabolic cost of inflammation, increases oxidative respiration, and improves exercise tolerance. Proc. Natl. Acad. Sci. USA 2017, 114, 2313–2318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmdahl, R.; Malissen, B. The need for littermate controls. Eur. J. Immunol. 2012, 42, 45–47. [Google Scholar] [CrossRef] [PubMed]
- Heiss, C.N.; Olofsson, L.E. Gut microbiota-dependent modulation of energy metabolism. J. Innate Immun. 2017. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Cai, X.; Liu, S.; Wang, S.; Nold-Petry, C.A.; Nold, M.F.; Bufler, P.; Norris, D.; Dinarello, C.A.; Fujita, M. Suppression of antigen-specific adaptive immunity by IL-37 via induction of tolerogenic dendritic cells. Proc. Natl. Acad. Sci. USA 2014, 111, 15178–15183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballak, D.B.; Li, S.; Cavalli, G.; Stahl, J.L.; Tengesdal, I.W.; van Diepen, J.A.; Kluck, V.; Swartzwelter, B.; Azam, T.; Tack, C.J.; et al. Interleukin-37 treatment of mice with metabolic syndrome improves insulin sensitivity and reduces pro-inflammatory cytokine production in adipose tissue. J. Biol. Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Laugerette, F.; Furet, J.P.; Debard, C.; Daira, P.; Loizon, E.; Geloen, A.; Soulage, C.O.; Simonet, C.; Lefils-Lacourtablaise, J.; Bernoud-Hubac, N.; et al. Oil composition of high-fat diet affects metabolic inflammation differently in connection with endotoxin receptors in mice. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E374–E386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.J.; Birtles, S.; de Schoolmeester, J.; Swales, J.; Moody, G.; Hislop, D.; O’Dowd, J.; Smith, D.M.; Turnbull, A.V.; Arch, J.R. Inhibition of 11β-hydroxysteroid dehydrogenase type 1 reduces food intake and weight gain but maintains energy expenditure in diet-induced obese mice. Diabetologia 2006, 49, 1333–1337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Netea, M.G.; Joosten, L.A.; Lewis, E.; Jensen, D.R.; Voshol, P.J.; Kullberg, B.J.; Tack, C.J.; van Krieken, H.; Kim, S.H.; Stalenhoef, A.F.; et al. Deficiency of interleukin-18 in mice leads to hyperphagia, obesity and insulin resistance. Nat. Med. 2006, 12, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Francesconi, W.; Sanchez-Alavez, M.; Berton, F.; Alboni, S.; Benatti, C.; Mori, S.; Nguyen, W.; Zorrilla, E.; Moroncini, G.; Tascedda, F.; et al. The proinflammatory cytokine interleukin 18 regulates feeding by acting on the bed nucleus of the stria terminalis. J. Neurosci. 2016, 36, 5170–5180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raedler, D.; Ballenberger, N.; Klucker, E.; Bock, A.; Otto, R.; Prazeres da Costa, O.; Holst, O.; Illig, T.; Buch, T.; von Mutius, E.; et al. Identification of novel immune phenotypes for allergic and nonallergic childhood asthma. J. Allergy Clin. Immunol. 2015, 135, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.C.; Li, H.M.; Wang, J.B.; Leng, R.X.; Wang, D.G.; Ye, D.Q. Elevated plasma interleukin-37 levels in systemic lupus erythematosus patients. Lupus 2016, 25, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Borges, M.C.; dos Santos Fde, M.; Telles, R.W.; Lanna, C.C.; Correia, M.I. Nutritional status and food intake in patients with systemic lupus erythematosus. Nutrition 2012, 28, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Locke, A.E.; Kahali, B.; Berndt, S.I.; Justice, A.E.; Pers, T.H.; Day, F.R.; Powell, C.; Vedantam, S.; Buchkovich, M.L.; Yang, J.; et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015, 518, 197–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zambon, A.; Hashimoto, S.I.; Brunzell, J.D. Analysis of techniques to obtain plasma for measurement of levels of free fatty acids. J. Lipid Res. 1993, 34, 1021–1028. [Google Scholar] [PubMed]
- Van Klinken, J.B.; van den Berg, S.A.; Havekes, L.M.; Willems Van Dijk, K. Estimation of activity related energy expenditure and resting metabolic rate in freely moving mice from indirect calorimetry data. PLoS ONE 2012, 7, e36162. [Google Scholar] [CrossRef] [PubMed]
- Weir, J.B. New methods for calculating metabolic rate with special reference to protein metabolism. J. Physiol. 1949, 109, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuipers, E.N.; Van Dam, A.D.; Ballak, D.B.; De Wit, E.A.; Dinarello, C.A.; Stienstra, R.; Van Diepen, J.A.; Rensen, P.C.N.; Boon, M.R. IL-37 Expression Reduces Lean Body Mass in Mice by Reducing Food Intake. Int. J. Mol. Sci. 2018, 19, 2264. https://doi.org/10.3390/ijms19082264
Kuipers EN, Van Dam AD, Ballak DB, De Wit EA, Dinarello CA, Stienstra R, Van Diepen JA, Rensen PCN, Boon MR. IL-37 Expression Reduces Lean Body Mass in Mice by Reducing Food Intake. International Journal of Molecular Sciences. 2018; 19(8):2264. https://doi.org/10.3390/ijms19082264
Chicago/Turabian StyleKuipers, Eline N., Andrea D. Van Dam, Dov B. Ballak, Ellemiek A. De Wit, Charles A. Dinarello, Rinke Stienstra, Janna A. Van Diepen, Patrick C.N. Rensen, and Mariëtte R. Boon. 2018. "IL-37 Expression Reduces Lean Body Mass in Mice by Reducing Food Intake" International Journal of Molecular Sciences 19, no. 8: 2264. https://doi.org/10.3390/ijms19082264