Abstract
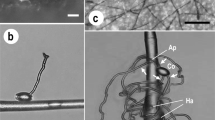
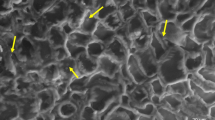
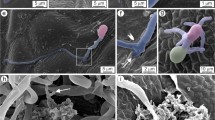
The present study aimed to explore the possibility of using the type I trichomes of tomato (Solanum lycopersicum) to monitor the infection processes of powdery mildews by microscopy. Individual trichome cells of two tomato genotypes were inoculated with pathogenic and non-pathogenic powdery mildew species, Pseudoidium neolycopersici, Erysiphe trifoliorum and Podosphaera xanthii. On the trichome cells of the tomato cultivar Moneymaker, hyphae of the pathogenic Pseudoidium neolycopersici (isolates KTP-03 and KTP-04) grew vigorously; whereas hyphal growth of the non-pathogenic Erysiphe trifoliorum and Podosphaera xanthii ceased after appressorium formation, which was associated with papilla formation and hypersensitive cell death, respectively. Similar infection processes of the tested powdery mildews were seen in Moneymaker epidermal cells. Therefore, tomato trichomes are suitable for analysing, at individual cell level, the infection processes of different pathotypes of powdery mildews and for observing the cytological responses of plants by non-pathogenic powdery mildews. On the other hand, it was observed that both isolates KTP-03 and KTP-04 failed to produce conidiophores on the hyphae elongating on Moneymaker trichomes. Similarly, no conidiophores were produced on the hyphae elongating on trichomes of Solanum peruvianum LA2172, which is resistant to KTP-03 and susceptible to KTP-04. Interestingly, delayed cell death occurred in LA2172 epidermal cells, which were attacked by KTP-03 hyphae elongating from trichomes and conidiophores were formed on new hyphae growing from the leaf epidermal cells. Thus, leaf trichomes and epidermal cells of the wild tomato species LA2172 reacted differently to the avirulent isolate KTP-03.





Similar content being viewed by others
References
Bai, Y., van der Hulst, R., Huang, C.-C., Wei, L., Stam, P., & Lindhout, P. (2004). Mapping Ol-4, a gene conferring resistance to Oidium neolycopersici and originating from Lycopersicon peruvianum LA2172, requires multi-allelic, single locus makers. Theoretical and Applied Genetics, 109, 1215–1223.
Chandran, D., Inada, N., Hather, G., Kleindt, C. K., & Wildermuth, M. C. (2010). Laser microdissection of Arabidopsis cells at the powdery mildew infection site reveals site-specific processes and regulators. Proceedings of the National Academy of Sciences of the United States of America, 107, 460−465.
Duffey, S. S. (1986). Plant glandular trichomes: Their partial role in defense against insects. In B. E. Juniper & T. E. Southwood (Eds.), Insects and the plant surface (pp. 151–172). London: Arnold.
Ellis, J. (2006). Insights into nonhost disease resistance: Can they assist disease control in agriculture? The Plant Cell, 18, 523–528.
Fujita, K., Matsuda, Y., Wada, M., Hirai, Y., Mori, K., Moriura, N., Nonomura, T., Kakutani, K., & Toyoda, H. (2004). Powdery mildew pathogens can suppress the chitinase gene expression induced in detached inner epidermis of barley coleoptile. Plant Cell Reports, 23, 504–511.
Glover, B. J. (2000). Differentiation in plant epidermal cells. Journal of Experimental Botany, 51, 497–505.
van der Hoorn, R. A. L., & Kamoun, S. (2008). From guard to decoy: A new model for perception of plant pathogen effectors. The Plant Cell, 20, 2009–2017.
Jones, J. D. G., & Dangl, J. L. (2006). The plant immune system. Nature, 444, 323–329.
Jones, H., Whipps, J. M., & Gurr, S. J. (2001). The tomato powdery mildew fungus Oidium neolycopersici. Molecular Plant Pathology, 2, 303–309.
Kang, J.-H., Liu, G., Shi, F., Jones, A. D., Beaudry, R. M., & Howe, G. A. (2010). The tomato odorless-2 mutant is defective in trichome-based production of diverse specialized metabolites and broad-spectrum resistance to insect herbivores. Plant Physiology, 154, 262–272.
Kashimoto, K., Matsuda, Y., Matsutani, K., Sameshima, T., Kakutani, K., Nonomura, T., Okada, K., Kusakari, S., Nakata, K., Takamatsu, S., & Toyoda, H. (2003). Morphological and molecular characterization for a Japanese isolate of tomato powdery mildew Oidium neolycopersici and its host range. Journal of General Plant Pathology, 69, 176–185.
Kennedy, G. G., & Sorenson, C. F. (1985). Role of glandular trichomes in the resistance of Lycopersicon hirsutum f. glabratum to Colorado potato beetle (Coleoptera: Chrysomelidae). Journal of Economic Entomology, 78, 547–551.
Kessler, A., & Baldwin, I. T. (2001). Defensive function of herbivore-induced plant volatile emissions in nature. Science, 291, 2141–2144.
Lemke, C. A., & Mutschler, M. A. (1984). Inheritance of glandular trichomes in crosses between Lycopersicon esculentum and L. pennellii. Journal of the American Society for Horticultural Science, 109, 592–596.
Lindeberg, M., Cunnac, S., & Collmer, A. (2012). Pseudomonas syringae type III effector repertoires: Last words in endless arguments. Trends in Microbiology, 20, 199–208.
Matsuda, Y., Kakutani, K., Nishimura, M., Wada, M., Nonomura, T., & Toyoda, H. (2003). Direct RT-PCR amplification of mature mRNAs in single trichome cells of plant leaves. Recent Research Developments in Cell Biology, 1, 145–153.
Matsuda, Y., Sameshima, T., Moriura, N., Inoue, K., Nonomura, T., Kakutani, K., Nishimura, H., Kusakari, S., Takamatsu, S., & Toyoda, H. (2005). Identification of individual powdery mildew fungi infecting leaves and direct detection of gene expression by single conidium polymerase chain reaction. Phytopathology, 95, 1137–1143.
McDowell, E. T., Kapteyn, J., Schmidt, A., Li, C., Kang, J.-H., Descour, A., Shi, F., Larson, M., Schilmiller, A., An, L., Jones, A. D., Pichersky, E., Soderlund, C. A., & Gang, D. R. (2011). Comparative functional genomic analysis of Solanum glandular trichome types. Plant Physiology, 155, 524–539.
Nonomura, T., Matsuda, Y., Xu, L., Kakutani, K., Takikawa, Y., & Toyoda, H. (2009a). Collection of highly germinatve pseudochain conidia of Oidium neolycopersici from conidiophores by electrostatic attraction. Mycological Research, 113, 364–372.
Nonomura, T., Xu, L., Wada, M., Kawamura, S., Miyajima, T., Nishitomi, A., Kakutani, K., Takikawa, Y., Matsuda, Y., & Toyoda, H. (2009b). Trichome exudates of Lycopersicon pennellii form a chemical barrier to suppress leaf-surface germination of Oidium neolycopersici conidia. Plant Science, 176, 31–37.
Nonomura, T., Nishitomi, A., Matsuda, Y., Soma, C., Xu, L., Kakutani, K., Takikawa, Y., & Toyoda, H. (2010). Polymorphic change of appressoria by the tomato powdery mildew Oidium neolycopersici on host tomato leaves reflects multiple unsuccessful penetration attempts. Fungal Biology, 114, 917–928.
Nonomura, T., Matsuda, Y., Takikawa, Y., Kakutani, K., & Toyoda, H. (2014). Successional changes in powdery mildew pathogens prevailing in common and wild tomato plants rotation-cultivated in a greenhouse. Annual Report of the Kansai Plant Protection Society, 56, 17–20.
Peter, A. J., & Shanower, T. G. (1998). Plant glandular trichomes: Chemical factories with many potential uses. Resonance, 3, 41–45.
Pichersky, E., & Gershenzon, J. (2002). The formation and function of plant volatiles: Perfumes for pollinator attraction and defense. Current Opinion in Plant Biology, 5, 237–243.
Sameshima, T., Kashimoto, K., Kida, K., Matsuda, Y., Nonomura, T., Kakutani, K., Nakata, K., Kusakari, S., & Toyoda, H. (2004). Cytological events in tomato leaves inoculated with conidia of Blumeria graminis f. sp. hordei and Oidium neolycopersici KTP-01. Journal of General Plant Pathology, 70, 7–10.
Schweizer, P. (2007). Nonhost resistance of plants to powdery mildew - new opportunities to unravel the mystery. Physiological and Molecular Plant Pathology, 70, 3–7.
Seifi, A., Kaloshian, I., Vossen, J., Che, D. D., Bhattarai, K. K., Fan, J. M., Naher, Z., Goverse, A., Tjallingii, W. F., Lindhout, P., Visser, R. G. F., & Bai, Y. (2011). Linked, if not the same, Mi-1 homologues confer resistance to tomato powdery mildew and root-knot nematodes. Molecular Plant-Microbe Interactions, 24, 441–450.
Seifi, A., Nonomura, T., Matsuda, Y., Toyoda, H., & Bai, Y. (2012). An avirulent tomato powdery mildew isolate induces localized acquired resistance to a virulent isolate in a spatiotemporal manner. Molecular Plant-Microbe Interactions, 25, 372–378.
Simmons, A. T., & Gurr, G. M. (2005). Trichomes of Lycopersicon species and their hybrids: Effects on pests and natural enemies. Agricultural and Forest Entomology, 7, 265–276.
Takikawa, Y., Kakutani, K., Nonomura, T., Matsuda, Y., & Toyoda, H. (2011). Conidia of Erysiphe trifoliorum attempt penetration twice during a two-step germination process on non-host barley leaves and an artificial hydrophobic surface. Mycoscience, 52, 204–209.
Takikawa, Y., Nonomura, T., Miyamoto, S., Okamoto, N., Murakami, T., Matsuda, Y., Kakutani, K., Kusakari, S., & Toyoda, H. (2015). Digital microscopic analysis of developmental process of conidiogenesis by powdery mildew pathogens isolated from melon leaves. Phytoparasitica, 43, 517–530.
Tooker, J. F., Peiffer, M., Luthe, D. S., & Felton, G. W. (2010). Trichomes as sensors detecting activity on the leaf surface. Plant Signaling and Behavior, 5, 73–75.
Acknowledgements
This work was supported by a Grant for Scientific Research from Faculty of Agriculture, Kindai University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attibution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Rights and permissions
About this article
Cite this article
Suzuki, T., Murakami, T., Takizumi, Y. et al. Trichomes: interaction sites of tomato leaves with biotrophic powdery mildew pathogens. Eur J Plant Pathol 150, 115–125 (2018). https://doi.org/10.1007/s10658-017-1257-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-017-1257-y