Abstract
Purpose
In low lying areas with dense networks of canals for land drainage, sediments accumulate in the waterways and have to be periodically dredged. These adjacent areas are mainly used for farming and agriculture and suffer from high rates of subsidence. The recycling of organic amendments, such as sediments, compost and manure, in agricultural soils can improve plant growth and yield, soil carbon content, and microbial biomass and activity, and have the potential to reverse the process of land subsidence.
Materials and methods
The effect of mixing bio-waste compost and the solid fraction of swine manure with dredged sediments before dewatering and biochemical ripening was investigated in terms of type and quantity of organic matter, CO2 production and O2 consumption, and N, P and S content. The water released during dewatering, the aggregate stability, and the undrained shear strength after ripening were also assessed since these areas have to be assessable by trucks and cattle.
Results and discussion
For the sediment with compost and manure the transformations in the type of organic matter, CO2 production and O2 consumption were larger compared to the individual fractions, indicating a positive priming effect. Most volume lost during ripening can be attributed to the loss of water and not to the loss of organic matter. In addition, the mixtures result in very stable aggregates and showed an undrained shear strength three times higher than measured for the sediments.
Conclusions
Sediments, compost and manure can be used and applied as beneficial use to reverse the process of land subsidence in low lying areas.
Similar content being viewed by others
1 Introduction
Soil organic matter (SOM), and the carbon (C) it contains, is associated with soil fertility for plant growth, soil and water quality, soil resistance to erosion, and thus stores at least three times more carbon than the atmosphere or living plants (Schmidt et al. 2011; Aich et al. 2013). The more stable fraction of SOM has a slower decomposition rate and slower release of nutrients than the more labile fractions of organic matter (Gruhn et al. 2000). The degradation of SOM significantly alters the soil texture and structure causing changes in soil hydrology and associated nutrient dynamics. Carbon (C), nitrogen (N), phosphorus (P), and sulphur (S) cycling are intimately linked through soil, plant and microbial processes affecting the C:N:P:S ratios of the soil organic matter (Kirkby et al. 2011).
In low lying delta areas with dense networks of canals, sediments accumulate in the waterways and have to be dredged periodically for several reasons, including the maintenance of the water quality, ecological status and navigability, and to prevent flooding (Querner et al. 2012). A beneficial use of the dredged sediments is to spread these on the land adjacent to the drained waterway. When non-impacted dredged sediments are transferred to upland conditions, the ripening process begins and transforms the waterlogged sediments into an aerated soil (Pons and Zonneveld 1965). During ripening, the previously anaerobic dredged sediments become aerobic which induces the oxidation of organic matter (Vermeulen et al. 2007). The oxidation of the organic matter can go in the direction of the humification or mineralization (Sanei et al. 2005). Humification can be defined as the transformation of SOM into amorphous humic compounds while mineralization refers to the transformation of organically bound elements (C, N, P, S) into inorganic compounds (Zech et al. 1997; Sumner 2000).
The recycling of organic amendments, such as dredged sediments, compost and manure, in agricultural soils can improve plant growth and yield, soil carbon content, and microbial biomass and activity (Thangarajan et al. 2013) and also have the potential to increase the surface level of the land in low lying polder areas which suffer from high rates of subsidence (Hoogland et al. 2012; Brils et al. 2014). There is evidence that the addition of organic amendments to soil can induce mineralization of the more stable fraction of the SOM and reduce the overall carbon content. This induced mineralization of the SOM is mainly due to the increase of SOM decomposing microorganisms which sustain after the initial organic amendment input is exhausted, and then survive on the remaining soil organic matter (Fontaine et al. 2004). This effect is called the positive priming effect and is mostly an acceleration of native SOM mineralization by using the organic amendment as a source of energy (Fontaine et al. 2011; Guenet et al. 2012). There are, however, also studies reporting a negative priming effect, i.e., the native SOM mineralization can be reduced (Kuzyakov et al. 2000). The positive or negative priming effect is mostly determined by the soil microbial biomass response to the addition of different substances (Kuzyakov et al. 2000).
The humification or mineralization of the organic matter also depends on the nutrients availability (Kirkby et al. 2014). Priming effects were found not only for C but also for N (Masayna et al. 1985; Fontaine et al. 2011; Dempster et al. 2012), P (Kuzyakov et al. 2000) and S (O’Donnell et al. 1994). The recycling of dredged sediments, compost and manure in soils can therefore have varying impacts, depending mainly on the soil type, composition of the organic amendment, climate conditions and period over which measurements are made (Zimmerman et al. 2011; Cely et al. 2014).
Even though there are many scientific references regarding the addition of compost and manure to soils, no literature was found regarding the mixture of compost and manure with dredged sediments. Therefore, the objective of this study is to evaluate the impact of adding compost of domestic bio-waste and the solid fraction of swine manure on the ripening of dredged sediments with the objective of doubling the organic matter content of the sediments. The impact was characterised in terms of type and quantity of organic matter, CO2 production and O2 consumption, and N, P and S content. The water released during dewatering, the aggregate stability, and the undrained shear strength after ripening were determined to evaluate the possibility of use of these materials in agriculture lands where tractors have to be used and cattle graze.
2 Material and methods
2.1 Collection of samples
The sediment (S) samples were dredged in November 2012 from a ditch surrounded by greenhouses and small family houses, located in Gouda, Zuid-Holland, the Netherlands. One container of 30 l was filled with sediment from the ditch using a bucket of 20 l for sampling. The air-tight container of 30 l was transported to the laboratory where the sediment was sieved and the fraction smaller than 2 mm was stored in the air-tight container at 4 °C until used in the experiments. The larger fractions were discarded. The 30 l container was in a roller bank for 24 h to homogenise before taking samples to use in the experiments.
The particle size distribution of the mineral fraction of the sediments was determined by first air-drying and then removing the organic matter with H2O2 and the CaCO3 with HCl. The particle size distribution was determined following the ISO standard ISO/TS 17892–4:2014. Fractions <2 μm, 2–16 μm, and 16–50 μm were determined by the pipet method and fraction 50–63 μm was determined by wet sieving at the Soil Chemical Biological Soil Laboratory (CBLB) of Wageningen UR. The fractions 63–105 μm, 105–150 μm, 150–210 μm, 210–420 μm, and 420–2000 μm were determined by dry sieving by the Soil Physics laboratory of Wageningen UR. The sediments have 25 % clay, 38 % silt, 37 % sand. The dredged sediments are classified as fine grained according to the ISO/TS 17892–4:2014 and classified as loam according to the USDA classification system of textural classes (USDA 1987).
The fibre content of the sediments was determined following the standard test method for laboratory determination of the fibre content of peat samples by dry mass ASTM D1997–91(2008). The fibre content of the sediments is 4.1 % gdry fibres/gdry matter.
The compost of domestic bio-waste (C) was provided by the Dutch company Attero. The domestic bio-waste was primarily separated at source and secondarily at Attero facilities. According to the Green Paper on the management of bio-waste in the European Union, “bio-waste is defined as biodegradable garden and park waste, food and kitchen waste from households, restaurants, caterers and retail premises, and comparable waste from food processing plants. It does not include forestry or agricultural residues, manure, sewage sludge, or other biodegradable waste such as natural textiles, paper or processed wood (COM 2008).
The solid fraction of swine manure (M) used in this research was provided by the Dutch company AquaPurga. To obtain the solid fraction, the manure was mixed with the copolymer of acrylamide and quaternized cationic monomer Zetag® 8140 and then the liquid and solid phases were mechanically separated using a belt press. The solid fraction used had a water content of 32 % (gwater g−1 sample). Sediment, C and M were stored at 4 °C in air-tight containers until used in the experiments.
2.2 Experimental procedure
2.2.1 Mixtures
The mixtures were prepared with the objective of doubling the organic matter content of the sediments. The mixtures of sediments with compost (S + C) and sediments with manure (S + M) were prepared considering the following ratios (in dry weight):
where mC is the dry mass of C; mS the dry mass of C; and mM the dry mass of M.
The ratios in Eqs. (1) and (2) were calculated from the water and organic matter content of S, C, and M before dewatering. The mixtures S + C and S + M were prepared before dewatering of the sediments, using a magnetic stirrer. The results of the mixtures S + C and S + M are later compared with the results that can be estimated from the controls S, C, and M, using Eqs. (1) and (2).
2.2.2 Dewatering
In order to dewater S, S + C and S + M, suction chambers (Vermeulen et al. 2005) were used where a suction pressure of 100 hPa was applied below the sample. To retain the fine particles in the suction chambers, a nylon filter with pore size of 0.45 μm was used. It was assumed that equilibrium between the matric potential of the sample and the applied suction is reached when the water outflow from the sample ceases (Klute and Dirksen 1986). This was generally observed within 7 days.
Dewatering was done at 20 ± 1 °C and the suction chambers were completely closed to the atmosphere, i.e., evaporation was avoided using a lid on the setup. Periodically a plug on top of the suction chamber was opened to release the suction pressure due to the decrease of the volume by removed water from the sample. The diameter of the dewatered samples was 6.0 ± 0.1 cm and the height of the S samples was 2.2 ± 0.2 cm, the height of the S + C samples was 3.3 ± 0.1 cm and the height of the S + M samples was 2.8 ± 0.1 cm.
The water content of samples C and M could not be decreased with a suction of 100 hPa, i.e., the samples were at a matric potential higher than 100 hPa. The C and M samples were moulded by hand to a final diameter of 6.0 ± 0.2 cm and a height of 3.3 ± 0.2 cm for C and 2.8 ± 0.2 cm for M, to come to equivalent size of samples as S + C and S + M, respectively. After dewatering, three replicates of each sample were sacrificed to determine the water and organic matter content and therefore to control the homogeneity between samples.
2.2.3 Biochemical ripening
After dewatering of S, S + C and S + M, and moulding C and M by hand, nine samples of each mixture and control were submitted to biochemical ripening in specifically designed batch bottles (BBs) (Fig. 1). Each sample was placed in one of these BBs which allowed monitoring of individual variations. The BBs were closed to the atmosphere and the gas phase was periodically monitored to assure that there was biological activity and that there was no oxygen limitation, i.e., oxygen concentration in the headspace was always above 10 % (v/v). To be able to take a gas sample, the BBs were designed with a gas sampling port. To avoid condensation and keep the relative humidity constant in the BBs, a saturated solution of potassium nitrate (KNO3) with a concentration of 360 g l−1 was kept inside each of the BBs. The KNO3 has neutral pH, absorbs about 0.03 % water in 80 % relative humidity over 50 days, and in a closed environment at 20 °C keeps the relative humidity constant at 94.5 %. Biochemical ripening was done during 37 days for S, and 35 days for C, M, S + C and S + M.
2.3 Analytical methods
Since the samples were analysed for different parameters at different phases of the experiment, the samples before dewatering will be referred to as phase a); after dewatering as phase b); and after biochemical ripening as phase c). The water removed from the samples during dewatering was collected in a tube for quantification by weighing. The moisture content and organic matter content were calculated from the oven dry weight of the samples (24 h at 105 °C) and the loss on ignition (4 h at 550 °C) of the samples (ISO/TS 17892–1:2014). Also O2 and CO2 in the BBs headspace were measured with gas chromatography (Shimadzu GC-2010) with a thermal conductivity detector (TCD). For practical reasons, this was done for all samples on the same day. Since not all BBs started on the same day, the gas measurements are not done on the same intervals for all the samples.
The pH of the S sample before dewatering was determined directly since water content was above 70 % for all samples. The pH of the C and M before ripening and S, C, M, S + C and S + M after biochemical ripening was determined on an a 1:1 sediment/manure/compost: water suspension using a glass electrode (Soil and Plant Analysis Council Inc. 1999).
The total N, P, and S were determined at the Chemical Biological Soil Laboratory of Wageningen University and Research Centre, The Netherlands. The samples were centrifuged to separate the solid fraction from the supernatant. The supernatant was then filtrated and the colloid fraction collected in the filter was added to the solid fraction. One sub-sample of the solid fraction was digested with the DigiPrep (aquaregia method, according to NEN-69,610) and analysed with a Thermo (type iCAP) ICP-AES. Another sub-sample of the solid fraction was used to analyse total N with a Leco C/N analyser. The total P and total S of the liquid fraction were analysed with a Varian ICP-AES and the total organic carbon and total nitrogen were analysed with a Skalar Segmented Flow Analyser (SFA).
The type of organic matter was characterised with the Rock-Eval 6® method. The samples of 20 mg of sediments are heated in pyrolysis and oxidation ovens at a heating rate of 25 °C min−1 of the Rock Eval 6® apparatus. The pyrolysis occurs between 100 and 650 °C under an inert atmosphere of N2. During the pyrolysis, the free hydrocarbons present in the sample and the hydrocarbons released by the thermal cracking of organic matter are detected by the Flame Ionisation Detector. Simultaneously, the CO and CO2 released during thermal cracking of oxygen-bearing organic compounds are measured by Infrared detectors. The measurement of CO is conducted up to 570 °C to avoid interference from the release of inorganic CO at higher temperatures. The measurement of CO2 is conducted up to 400 °C to avoid interference from the release of inorganic CO2 at higher temperatures. Following the pyrolysis stage, the sample is transferred to the oxidation oven and heated from 400 to 850 °C in air, incinerating all the residual organic carbon. The CO and CO2 released during combustion of residual organic carbon are measured by online Infrared detectors. The HI (Hydrogen Index) is an indicative value for the amount of hydrocarbons released by thermal cracking of organic matter per gramme of TOC (Total Organic Carbon), and the OIRE6 (Oxygen Index) is an indicative value for the amount of CO2 and CO by thermal cracking of oxygen-bearing organic compounds per gramme of TOC (Electronic Supplementary Material, Table S1). Changes in the HI and OIRE6 are analysed to evaluate a transition in the type of organic matter. Rock-Eval 6® analyses were performed at Deltares, the Netherlands.
Aggregate stability was determined according to the method described by Kemper and Rosenau (1986) for wet aggregate stability determination. This method measures the breakdown of aggregates upon rapid wetting (slaking) and the subsequent breakdown of aggregates due to the mechanical sieving action. The wet sieving apparatus from Eijkelkamp was used for the mechanical sieving. The aggregate stability index goes from 0 to 1, where 1 is very stable and 0 is absence of aggregates (Kemper and Rosenau 1986).
The undrained shear strength was determined with the fall cone method of the biochemically ripened samples, following the procedure described in the ISO/TS 17892–6:2004. The measured undrained shear strengths were used to calculate the bearing capacity in order to evaluate whether the soil is accessible for cattle and tractors. The bearing capacity (q) of the soil is calculated according to the method described e.g. in Terzaghi et al. (1996):
in which q is the bearing capacity in [kPa], cu is the undrained shear strength in [kPa], γ is the bulk unit weight of the soil in [kN m−3], D is the depth at which the load is applied below surface level in [m], B, is the width of the load surface in [m] assuming an infinitely long strip footing, Nc, Nq and Nγ are so-called bearing capacity factors, which are related the friction angle of the soil, sc, sq. and sγ are shape factors which are used to correct for the shape of the surface on which the load is applied, dc and dq are depth factors which need to be taken into account when the load is applied below surface level. Considering that the load of cattle or tractors is applied at surface level and only for a short period of time at the same location, D = 0 m, Nγ = 0, Nq = 1 and Nc = 5.14 (Barnes 2000), the equation above can be simplified to:
In this empirical approach the soil is presumed to be infinitely stiff and homogeneous in both horizontal and vertical directions, which are considered to be valid presumptions when the load is applied in the open field. In case a tractor or cow is standing close to a ditch the bearing capacity may be significantly lower and that scenario was not considered in this study.
The load applied by cattle on the soil was calculated considering an average weight of 600 kg, and considering that the weight is evenly distributed by the 4 hoofs with a surface area of 0.01 m2 each. For tractors, it was considered an average weight of 2900 kg per front wheel and an average weight of 6000 kg per rear wheel (Table 1).
3 Results
In all samples the Total Organic Carbon, TOC (RC + PC), represents more than 70 % of the Total Carbon, TC (TOC + MINC), for all samples, both before dewatering (a) and after biochemical ripening (c) (Fig. 2). The TOC of S + Ca) and S + Ma) is approximately 2 times the TOC of Sa), as desired with the mixtures. During biochemical ripening, the decrease in TOC was 26 % for S + C, 10 % for S + M, and 25 % for S. The decrease in MINC was 39 % for S + C, 42 % for S + M, and 51 % for S (Fig. 2). The estimated TOC for S + Cc) from Sc) and Cc) (using Eq. (1)) is 6 % higher than the measured and the estimated TOC for S + Mc) from Sc) and Mc) (using Eq. (2)) is 9 % smaller than the measured.
During biochemical ripening, PC decreased 28 % for S + C and 5 % for S + M which is less than the 31 % decrease for S (Fig. 2). RC decreased 25 % for S + C and 12 % for S + M while S had a decrease of 23 % (Fig. 2). The estimated PC for S + Cc) from Sc) and Cc) (using Eq. (1)) was similar to the measured, and the estimated PC for S + Mc) from Sc) and Mc) (using Eq. (2)) is 28 % smaller than the measured. The estimated RC for S + Cc) from Sc) and Cc) (using Eq. (1)) was 9 % higher than the measured and the estimated RC for S + Mc) from Sc) and Mc) (using Eq. (2)) was 2 % higher than the measured.
The type of organic matter by Rock Eval analysis showed before dewatering, S + Ca) and S + Ma) had similar HI and S + Ca) had higher OIRE6 than S + Ma). After biochemical ripening, S + C had 1 % increase in HI and 20 % decrease in OIRE6, and S + M had 46 % increase in HI and 25 % decrease in OIRE6, while S had 16 % increase in HI and 12 % decrease in OIRE6. Therefore, S + C, S + M and S all had an increase in HI and a decrease in OIRE6. The control C had a decrease in HI and decrease in OIRE6. The control M had a decrease in HI and increase in OIRE6 and Ma) was the sample with the highest HI and lowest OIRE6 (Fig. 3).
The HI of S + Cc) was 4 % smaller than the estimated from Sc) and Cc) (using Eq. (1)) while the HI for S + Mc) was 30 % higher than the estimated from Sc) and Mc) (using Eq. (2)). The OIRE6 for S + Cc) and S + Mc) was 19 % and 16 % smaller, respectively, than the estimated from the individual parts Sc), Cc) and Mc).
The TOC content before dewatering was used to normalise the results of CO2 production and O2 consumption during biochemical ripening (Fig. 4). The results in Fig. 4 confirm that there was microbial activity in the BBs and consistently show a higher CO2 production than O2 consumption. The mixtures S + C and S + M result in higher CO2 production and O2 consumption per gramme of TOC than the observed for the controls S, C and M. The mixture S + M had higher total CO2 production and O2 consumption per gramme of TOC than the mixture S + C. The O2 consumption of S + C and S + M was similar until 15 days and after that S + M had higher O2 consumption than S + C (Fig. 4). The molar ratio [CO2]:[O2] was almost constant with time for C and M, and increased slightly with time for S, S + C and S + M (Electronic Supplementary Material, Fig. S1).
The carbon mass balance (Table 2) has a gap of less than 8 % for S, C, M and S + M and a gap of 14 % for S + C. The carbon mass balance is not completely closed probably due to leakages in the BBs, i.e., the carbon released to the atmosphere during biochemical ripening was higher than the measured, and due to transport of atmospheric air to the BBs when sampling (invasive sampling method).
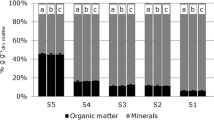
Despite the decrease in TOC observed with the Rock-Eval method (Fig. 2), the organic matter content determined by loss on ignition did not show a significant change during dewatering and biochemical ripening (Fig. 5). The standard deviation of the organic matter content (Fig. 5) confirmed that the replicates were homogeneous.
Analysis of nutrients and pH in Table 3 showed that Total Nitrogen (TN) decreased 18 % for S + C and 22 % for S + M even though TN did not significantly change for the control samples S, C and M. For Total Phosphorus (TP) and Total Sulphur (TS) all changes were less than 7 % (Table 3). The pH (Table 3) did not significantly change during biochemical ripening. The volume lost during dewatering was 34 ± 3 % for S + C, 47 ± 4 % for S + M and 49 ± 3 % for S. All volume lost was due to water removal since no significant decrease in organic matter content was observed.
Dewatering showed that most water was removed from the samples within the initial 48 h (Fig. 6A). S + C had less of its total initial water content removed during dewatering than S alone (Fig. 6A). This was in line with the lower water content of C in relation to S (Fig. 6B).
From Fig. 6B, dewatering led to a significant loss of total water for S, S + C, and S + M while during biochemical ripening the water content did not significantly change for any sample as the matric potential was set to 100 hPA. The water content of C and M was not removable with a suction of 100 hPa and therefore these samples were not submitted to dewatering in the suction chambers.
The aggregate stability index of the fraction 2–4 mm is slightly higher than the fraction 1–2 mm (Table 4). The addition of C and M to S resulted in significantly higher undrained shear strength (Table 4) for S + C and S + M than for S. While for S the undrained shear strength after dewatering and biochemical ripening was not enough to sustain cattle or tractors (Table 1), for S + C and S + M it was enough. The undrained shear strength could not be determined for samples C and M since these had no structure.
4 Discussion
The total organic carbon (TOC) represents more than 70 % of the total carbon (TC) for all samples, both before dewatering and after biochemical ripening. During biochemical ripening, the TC decreased less for the mixtures S + C and S + M than for the control S. Both fractions of TC, TOC and MINeral Carbon (MINC), decreased during biochemical ripening and the correlation coefficient between the decrease in TOC and the decrease in MINC is 0.56, indicating no strong correlation. The discussion of the results focus on TOC not only because it represents more than 70 % of the TC but also because this is the most reactive fraction to environmental changes such as temperature, moisture, oxygen levels, pH, and nutrients availability (Coleman et al. 2004). The objective of adding Compost (C) or Manure (M) to Dredged sediments (S) was to double the organic matter content of the sediments and the TOC of S + Ca) and S + Ma) confirms that this was achieved. The addition of compost to sediments results in higher decrease in TOC than the addition of manure (Electronic Supplementary Material, Table S2), i.e., the addition of compost and manure have different effects on the behaviour of sediments during biochemical ripening.
Regarding the RC and PC, the mixture S + C has similar behaviour as S but the mixture S + M results in less decrease in both RC and PC than S. Furthermore, RC decreased more for S + C than the estimated from the controls, and PC decreased less for S + M than the estimated from the controls (Electronic Supplementary Material, Table S2). All samples had higher RC (oxidizable organic carbon) than PC (pyrolysable hydrocarbons), both before dewatering and after biochemical ripening. RC represents a more stable fraction of organic carbon than PC.
Both S + C and S + M had an increase in HI and a decrease in OIRE6 during biochemical ripening which indicates that the mixtures resulted in the mineralization of organic matter. The mixture S + C had smaller decrease in HI than S while S + M had higher decrease in HI than S, which indicates that the addition of M to S results in higher rate of mineralization while the addition of C to S results in lower rate of mineralization. Both S + C and S + M had higher decrease in OIRE6 than S which indicated that both the addition of C and M to S result in higher rates of mineralization of oxygen-bearing compounds (Electronic Supplementary Material, Table S2).
Also Ma) had the highest TOC and HI, and lowest OIRE6 content before dewatering, indicating that this was the sample with highest content of easily degradable organic matter. During biochemical ripening, M had the smallest decrease in TOC, had a decrease in PC, was the only with an increase in RC, and was the only sample with a decrease in HI and increase in OIRE6, which indicates that humification occurred for the control M.
The mixtures S + C and S + M resulted in higher CO2 production and O2 consumption per gramme of TOC than the controls S, C and M, i.e., there was a positive priming effect. The mixture S + M had higher total CO2 production and O2 consumption per gramme of TOC than the mixture S + C, which goes in line with the higher decrease of HI for S + M than S + C. Both mixtures resulted in higher decrease in PC than could be expected from the emissions of the individual parts. The mixture S + M had significantly higher decrease in PC than estimated from the individual parts. These results are indicative of a positive priming effect, i.e., there is more CO2 production and O2 consumption during the biochemical ripening of the mixtures S + C and S + M than during the biochemical ripening of the controls S, C, and M. Furthermore, the HI increased much more for S + M than estimated from the controls, and the OIRE6 decreased more for S + C and S + M than estimated from the controls (Electronic Supplementary Material, Table S2). Therefore, the addition of M to S seems to enhance the increase in hydrocarbons and decrease in oxygen-bearing compounds which is also indicative of a positive priming effect. Other authors found that the magnitude and direction (positive or negative) of the priming effect depend on the soil type, type of organic matter added and also on the period over which measurements are made (Zimmerman et al. 2011; Cely et al. 2014). The results of this research confirm the mixture S + M results in more changes in the type of organic matter than the mixture S + C. When transferring the sediments, compost and manure to the experimental conditions, the equilibrium of the organic matter of the three amendments is disturbed and transformations occur until a new equilibrium is reached (Coleman et al. 2004). Since the CO2 production and O2 consumption did not achieve a constant rate, a longer experiment could give more information.
The molar ratio [CO2]:[O2], also designed as respiratory quotient, ranged from 0.63 for S and M to 0.91 for S + M, suggesting that aliphatic organic compounds, amino acids, or refractory components containing relatively little oxygen were predominantly mineralised (Dilly 2001).
The determination of the organic matter content of the samples using the loss on ignition (LOI) method did not show a significant change during dewatering and biochemical ripening. This was also observed in previous studies where biochemical ripening of dredged sediments, with similar organic matter content and particle size distribution, was performed for longer periods (Oliveira et al., 2016). The standard deviation of organic matter content determined by the LOI method was used as a confirmation about the homogeneity among the replicates.
Total nitrogen decreased 18 % for S + C and 22 % for S + M, suggesting there was a positive priming effect for S + C and S + M (Kuzyakov et al. 2000), i.e., the mixtures resulted in higher decrease in TN than the controls. For TP and TS, the changes were less than 7 % and therefore can be considered negligible.
The mixture S + M had higher CO2 production and O2 consumption per gramme of organic matter than the mixture S + C which can be related with the higher TN and TP content, indicating the role that nutrients have in microbial activity. This contradicts the hypothesis that the priming effect is lower when nutrients availability is higher (Fontaine et al. 2011).
Some previous studies with soils amended with swine manure found a positive priming effect (Bernal and Kirchmann 1992; Kotzerke et al. 2008; Angers et al. 2010). Even though, a previous study with a loamy soil with 8 mgC/gsoil and 0.9 mgN/gsoil amended with swine manure with 263 mgC/g and 27.3 mgN/g, did not find a significant change in the CO2 dynamics between the control soil and the amended soil (Dendooven et al. 1998), evidencing that different initial C and N content result in different priming effects. Different studies have shown that the addition of compost to soils, including soils with organic contaminants, increases the rate of C and N mineralization (Hupe et al. 1996; Borken et al. 2002; Gandolfi et al. 2010). The results of this research confirm that the impact of compost and manure is highly variable since it depends on the type of the organic matter, nutrients content and maturity of the material.
The volume lost during dewatering was 49 ± 3 % for S, 34 ± 3 % for S + C and 47 ± 4 % for S + M. All volume lost was due to water removal since no significant decrease in organic matter content was observed. From this result, we can attribute the loss in volume to water loss and compaction and not to organic matter oxidation as reported in a previous study (Schothorst 1977).
Most water was removed within the initial 48 h. The initial geotechnical water content of S was more than 350 % (mass of water per mass of dry sample) due to the sampling method and the natural dewatering could take several weeks (Vermeulen et al. 2003; Zentar et al. 2011). During biochemical ripening the water content did not significantly change for any sample since a saturated salt solution of KNO3 was used to keep the relative humidity constant and to avoid condensation.
The aggregates formed during biochemical ripening are very stable to water erosion. The fraction 2–4 mm has slightly more stable aggregates than the fraction 1–2 mm. An explanation for this is that soil aggregates protect occluded organic matter against degradation (van Lützow et al. 2006; Chaplot and Cooper 2015). Improvement of aggregate stability was confirmed when repeated application of compost and manure to soil increased the aggregate stability (Annabi et al. 2011).
The mixtures S + C and S + M resulted in an undrained shear strength higher than S alone. While for S the undrained shear strength after dewatering and biochemical ripening was not enough to sustain cattle or tractors (Table 1), for S + C and S + M it was enough. Therefore, the addition of C and M might also decrease the period between spreading the sediments and re-using the land.
5 Conclusions
Most volume lost during ripening can be attributed to the loss of water and not to the loss of organic matter, indicating that spreading sediments, compost and manure to agriculture soils is a suitable approach to reverse the process of land subsidence. The results of this research confirm that the impact of compost and manure on the ripening of dredged sediments is highly variable since it depends on the type of the organic matter, nutrients content and maturity of the material. The results for the control manure indicate a humification of the organic matter, with transformation of the organic matter into amorphous humic compounds, while the results for all other samples indicate a mineralization of the organic matter, with transformation of organic matter into inorganic compounds. The results indicate that more changes in the type of organic matter occur when sediments are mixed with compost or manure fraction than it could be expected from the changes in the individual parts, which indicated a positive priming effect. Since the CO2 production and O2 consumption were still ongoing, a longer duration of the biochemical ripening experiment could give more information about the long term effect of adding compost or manure to sediments. Using suction to dewater the samples, most dewatering occurs within the first 48 h which allows for short term experiments on a lab scale. After biochemical ripening, the control sample of sediments and the mixtures of sediments and compost or manure are very resistant to water erosion which is indicated by the high aggregate stability of the samples. The mixtures resulted in and undrained shear strength 3 times higher than the measured for the control S which is an important improvement compared to the regular spreading of sediments on land and reduces the time between application of sediments and re-use of the land.
References
Aich S, McVoy CW, Dreschel TW, Santamaria F (2013) Estimating soil subsidence and carbon loss in the Everglades agricultural area, Florida using geospatial techniques. Agric Ecosyst Environ 171:124–133
Angers DA, Chantigny MH, MacDonald JD, Rochette P, Côté D (2010) Differential retention of carbon, nitrogen and phosphorus in grassland soil profiles with long-term manure application. Nutr Cycl Agro Eco Sys 86:225–229
Annabi M, Le Bissonnais Y, Le Villio-Poitrenaud M, Houot S (2011) Improvement of soil aggregate stability by repeated applications of organic amendments to a cultivated silty loam soil. Agric Ecosyst Environ 144:382–389
Barnes GE (2000) Soil mechanics-Principles and practice. PALGRAVE
Bernal MP, Kirchmann H (1992) Carbon and nitrogen mineralization and ammonia volatilization from fresh, aerobically and anaerobically treated pig manure during incubation with soil. Biol Fert Soils 13:135–141
Borken W, Muhs A, Beese F (2002) Application of compost in spruce forests: effects on soil respiration, basal respiration and microbial biomass. Forest Ecol Manag 159:49–58
Brils J, de Boer P, Mulder J, de Boer E (2014) Reuse of dredged material as a way to tackle societal challenges. J Soils Sediments 14:1638–1641
Cely P, Tarquis AM, Paz-Ferreiro J, Méndez A, Gascó G (2014) Factors driving the carbon mineralization priming effect in a sandy loam soil amended with different types of biochar. Solid Earth 5:585–594
Chaplot V, Cooper M (2015) Soil aggregate stability to predict organic carbon outputs from soils. Geoderma 243–244:205–213
Coleman DC, Crossley DA Jr, Hendrix PF (2004) Fundamentals of soil ecology. Elsevier Academic, Amsterdam, NL
COM (2008) Green Paper On the management of bio-waste in the European Union COM (2008) 811 Final
Dempster DN, Gleeson DB, Solaiman ZM, Jones DL, Murphy DV (2012) Decreased soil microbial biomass and nitrogen mineralisation with eucalyptus biochar addition to a coarse textured soil. Plant Soil 354:311–324
Dendooven L, Bonhomme E, Merckx R, Vlassak K (1998) Injection of pig slurry and its effects on dynamics of nitrogen and carbon in a loamy soil unter laboratory conditions. Biol Fert Soils 27:5–8
Dilly O (2001) Microbial respiratory quotient during basal metabolism and after glucose amendment in soils and litter. Soil Biol Biochem 33:117–127
Fontaine S, Bardoux G, Abbadie L, Mariotti A (2004) Carbon input to soil may decrease soil carbon content. Ecol Lett 7:314–320
Fontaine S, Henault C, Aamor A, Bdioui N, Bloor JMG, Maire V, Mary B, Revaillot S, Maron PA (2011) Fungi mediate long term sequestration of carbon and nitrogen in soil through their priming effect. Soil Biol Biochem 43:86–96
Gandolfi I, Sicolo M, Franzetti A, Fontanarosa E, Santagostino A, Bestetti G (2010) Influence of compost amendment on microbial community and ecotoxicity of hydrocarbon-contaminated soils. Bioresource Technol 101:568–575
Gruhn P, Goletti F, Yudelman M (2000) Food, Agriculture, and the Environment Discussion Paper 32, International Food Policy Research Institute
Guenet B, Juarez S, Bardoux G, Abbadie L, Chenu C (2012) Evidence that stable C is as vulnerable to priming effect as is more labile C in soil. Soil Biol Biochem 52:43–48
Hoogland T, van den Akker JJH, Brus DJ (2012) Modeling the subsidence of peat soils in the Dutch coastal area. Geoderma 171–172:92–97
Hupe K, Lüth JC, Heerenklage J, Stegmann R (1996) Enhancement of the biological degradation of soils contaminated with oil by the addition of compost. Acta Biotechnol 16:19–30
Kemper WD, Rosenau RC (1986) Aggregate stability and size distribution. In: Klute A (ed) Methods of soil analysis. Part 1- physical and mineralogical methods. Second edition. American Society of Agronomy, Inc. and Soil science Society of America, Inc, Madison, Wisconsin USA, USA, pp. 425–442
Kirkby CA, Kirkegaard JA, Richardson AE, Wade LJ, Blanchard C, Batten G (2011) Stable soil organic matter: a comparison of C:N:P:S ratios in Australian and other world soils. Geoderma 163:197–208
Kirkby CA, Richardson AE, Wade LJ, Passioura JB, Batten GD, Blanchard C, Kirkegaard JA (2014) Nutrient availability limits carbon sequestration in arable soils. Soil Biol Biochem 68:402–409
Klute A, Dirksen C (1986) Water retention: laboratory methods. In: Klute A (ed) Methods of soil analysis. Part 1- physical and mineralogical methods, 2nd edn. American Society of Agronomy, Inc. and Soil science Society of America, Inc, Madison, Wisconsin USA, Wisconsin, USA, pp. 635–686
Kotzerke A, Sharma S, Schauss K, Heuer H, Thiele-Bruhn S, Smalla K, Wilke B-M, Schloter M (2008) Alterations in soil microbial activity and N-transformation processes due to sulfadiazine loads in pig-manure. Environ Poll 153:315–322
Kuzyakov Y, Friedel JK, Stahr K (2000) Review of mechanisms and quantification of priming effects. Soil Biol Biochem 32:1485–1498
Masayna W, Kai H, Kawaguchi S (1985) Nitrogen behavior in tropical wetland rice soils. 2. The efficiency of fertilizer nitrogen, priming effect and A-values. Fert Res 6:37–47
O’Donnell AG, Wu J, Syers JK (1994) Sulphate-S amendments in soil and their effects on the transformation of soil Sulphur. Soil Biol Biochem 26:1507–1514
Oliveira BRF, Smit MPJ, van Paassen LA, Grotenhuis JTC, Rijnaarts HHM (2016) Functional properties of soils formed from biochemical ripening of dredged sediments – subsidence mitigation in delta areas. J Soils Sediments. doi:10.1007/s11368-016-1570-7
Pons LJ, Zonneveld IS (1965) Soil ripening and soil classification: initial soil formation of alluvial deposits with a classification of the resulting soils, Publication 13, International Institute for Land Reclamation and Improvement
Querner EP, Jansen PC, van den Akker JJH, Kwakernaak C (2012) Analysing water level strategies to reduce soil subsidence in Dutch peat meadows. J Hydrol 446–447:59–69
Sanei H, Stasiuk LD, Goodarzi F (2005) Petrological changes occurring in organic matter from recent lacustrine sediments during thermal alteration by rock-Eval pyrolysis. Org Geochem 36:1190–1203
Schmidt MWI, Torn MS, Abiven S, Dittmar T, Guggenberger G, Janssens IA, Kleber M, Kogel-Knabner I, Lehmann J, Manning DAC, Nannipieri P, Rasse DP, Weiner S, Trumbore SE (2011) Persistence of soil organic matter as an ecosystem property. Nature 478:49–56
Schothorst CJ (1977) Subsidence of low moor peat soils in the western Netherlands. Geoderma 17:265–291
Soil and Plant Analysis Council Inc (1999) Soil analysis handbook of reference methods. CRC Press LLC
Sumner ME (2000) Handbook of soil science. CRC Press, Boca Raton, FL, USA
Terzaghi K, Peck RB, Mesri G (1996) Soil mechanics in engineering practice. Wiley-Interscience, ISBN 0471086584
Thangarajan R, Bolan NS, Tian G, Naidu R, Kunhikrishnan A (2013) Role of organic amendment application on greenhouse gas emission from soil. Sci Total Environ 465:72–96
USDA (1987) Soil Mechanics Level 1, Module 3- USDA Textural Classification Study Guide, National Employee Development Staff, Soil conservation Service, United States Department of Agriculture
van Lützow M, Kögel-Knabner I, Ekschmitt K, Matzner E, Guggenberger G, Marschner B, Flessa H (2006) Stabilization of organic matter in temperate soils: mechanisms and their relevance under different soil conditions – a review. Eur J Soil Sci 57:426–445
Vermeulen J, Grotenhuis T, Joziasse J, Rulkens W (2003) Ripening of clayey dredged sediments during temporary upland disposal a bioremediation technique. J Soils Sediments 3:49–59
Vermeulen J, van Dijk SG, Grotenhuis JTC, Rulkens WH (2005) Quantification of physical properties of dredged sediments during physical ripening. Geoderma 129:147–166
Vermeulen J, van Gool MPM, Dorleijn AS, Joziasse J, Bruning H, Rulkens WH, Grotenhuis JTC (2007) Biochemical ripening of dredged sediments. Part 1. Kinetics of biological organic matter mineralization and chemical sulfur oxidation. Environ Toxicol Chem 26:2530–2539
Zech W, Senesi N, Guggenberger G, Kaiser K, Lehmann J, Miano TM, Miltner A, Schroth G (1997) Factors controlling humification and mineralization of soil organic matter in the tropics. Geoderma 79:117–161
Zentar R, Miraoui M, Abriak NE, Benzerzour M (2011) Natural dewatering of marine dredged sediments. Dry Technol 29:1705–1713
Zimmerman AR, Gao B, Ahn M-Y (2011) Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol Biochem 43:1169–1179
Further reading
Lafargue E, Marquis F, Pillot D (1998) Rock-Eval 6 applications in hydrocarbon exploration, production, and soil contamination studies. Oil Gas Sci Technol 53:421–437. This is mentioned in the electronic supplementary material Table S1
Acknowledgments
This research is supported by the Dutch Technology Foundation STW, which is part of the Netherlands Organisation for Scientific Research (NWO), and which is partly funded by the Ministry of Economic Affairs, via the perspective program BioGeoCivil (grant 11344). We would like to express gratitude to Guido Verweij from Hoogheemraadschap van Schieland en de Krimpenerwaard (HHSK) for supporting with sampling of sediments; Adrie Veeken from Attero for providing compost; and Maria Briglia from Aqua Purga for proving the manure fraction.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Jos Brils
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Oliveira, B.R.F., van Laarhoven, K., Smit, M.P.J. et al. Impact of compost and manure on the ripening of dredged sediments. J Soils Sediments 17, 567–577 (2017). https://doi.org/10.1007/s11368-016-1571-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-016-1571-6