- AHP
activity-related heat production
- OTH
oat hulls
- RS
resistant starch
- SBP
sugarbeet pulp
- WB
wheat bran
The use of dietary fibre has gained much interest during the past decades, in both animal and human nutrition. In human subjects dietary fibres have been studied intensively for their possible effects on health (e.g. constipation, diabetes mellitus, CVD) and body-weight management. Initially, the interest of animal nutritionists in dietary fibre sources was related to their availability as cheap byproducts from food production (e.g. wheat straw, oat hulls (OTH), soyabean hulls, sugarbeet pulp (SBP)). In past decades animal researchers have become increasingly aware that in addition to providing nutrients to animals dietary ingredients can have broader effects, such as stimulating gut health and improving well-being in general. Dietary fibre has been observed to reduce behavioural problems that are, at least in part, related to insufficient satiety in restrictedly-fed animals (e.g. breeding sows(Reference Meunier-Salaün, Edwards and Robert 1 ) and broiler breeders(Reference Zaczek, Jones, Macleod and Hocking 2 )). Apart from stimulating nutritional satiety, dietary fibre may also satisfy specific behavioural needs related to natural feeding habits such as rooting and chewing in pigs(Reference Meunier-Salaün, Edwards and Robert 1 ), food pecking in chickens(Reference Van Krimpen, Kwakkel, Reuvekamp, Van Der, Den Hartog and Verstegen 3 ) and rumination in calves(Reference Redbo and Nordblad 4 ). Since the early 1990s the interest in using dietary fibre in diets of restrictedly-fed breeding sows has increased, particularly as several studies have reported positive effects of dietary fibre on behaviour and welfare. Satiety per se was not always measured systematically in these studies, and data on voluntary feed intake of fibre sources are often lacking. The studies that have been performed in sows might, however, provide valuable insight into the effects of specific characteristics of different fibre sources on behaviours that might reflect satiety.
Thus, the aim of the present paper is to review effects of dietary fibre on behaviour and satiety in pigs. First, relationships between behaviour and satiety will be described. Then, hypotheses will be formulated about the relationships between specific characteristics of dietary fibre sources and behaviours that might reflect satiety. Finally, the validity of these hypotheses will be examined using available literature sources.
Relationships between behaviour and satiety
Although domesticated pigs are genetically altered as a result of intensive breeding programmes, their behavioural repertoire still largely resembles that of their wild ancestors and relatives(Reference Stolba and Wood-Gush 5 ). Under (semi-)natural conditions both wild and domestic pigs are non-specialist omnivores that can exploit an enormous range of food sources(Reference Schley and Roper 6 ) and forage for patchily-distributed food(Reference Gundlach 7 ). Typically, bouts of rooting and nosing at the ground are separated by locomotion to different foraging areas. As appetitive and consummatory phases may thus be interspersed over long periods, appetitive behaviour is probably stimulated by the ingestion of food(Reference Terlouw, Lawrence and Illius 8 , Reference Wiepkema 9 ) until satiety signals from the body provide a negative feedback on feed intake(Reference Hughes and Duncan 10 ). Under semi-natural conditions pigs spend most of the day engaged in foraging-related behaviours(Reference Stolba and Wood-Gush 5 ). In intensive husbandry, however, pigs are mostly kept in barren environments in which the opportunities for expressing these evolutionary established foraging behaviours are limited. Moreover, in non-lactating breeding sows feed allowance is limited in order to prevent excessive fatness and reduced reproductive performance. Although the animals are provided with sufficient nutrients for maintenance and reproduction, traditional diets are consumed within minutes (e.g. 11·4 min, single meal per d(Reference Brouns, Edwards and English 11 ); 7·6 min, two meals per d(Reference Bergeron, Bolduc, Ramonet, Meunier-Salaun and Robert 12 )) and the feeding motivation of the sows remains high(Reference Lawrence, Appleby and MacLeod 13 ). In combination with the barren stimulus-poor environment this factor may lead to the channelling of natural foraging behaviours into a few simple behavioural elements, like bar-biting and sham chewing(Reference Lawrence and Terlouw 14 ). When these abnormal behaviours are shown repetitively, unvarying and apparently without function, they are called stereotypies(Reference Ödberg 15 ). Stereotypies are associated with the secretion of endorphins, which play a role in adaptation to stress(Reference Cronin 16 ).
Behaviour reflecting immediate and prolonged postprandial satiety
Stereotypies (also often referred to as self-directed behaviour or non-feeding oral activities) are displayed in close relationship with a meal, and particularly shortly after feeding(Reference Terlouw, Wiersma, Lawrence and MacLeod 17 ). As both feeding levels and fibrous diets have been shown to effectively reduce these behaviours after a meal (for example, see Terlouw et al. (Reference Terlouw, Lawrence and Illius 8 ); Brouns et al. (Reference Brouns, Edwards and English 11 ); Bergeron et al. (Reference Bergeron, Bolduc, Ramonet, Meunier-Salaun and Robert 12 ); Ramonet et al. (Reference Ramonet, Robert, Aumaître, Dourmad and Meunier-Salaün 18 )) post-meal stereotypies may reflect (lack of sufficient) satiety. When rooting material is present on the floor, which partly allows the expression of natural foraging behaviour, substrate-directed rooting and nosing may also be used as immediate postprandial indicators of satiety. Several studies report increased rooting when access to feed is restricted in pigs(Reference Beattie and O'Connell 19 –Reference Jensen, Kyriazakis and Lawrence 21 ). As manipulation of pen components seems to replace manipulation of rooting material in its absence(Reference De Leeuw and Ekkel 22 , Reference Whittaker, Spoolder, Edwards, Lawrence and Corning 23 ), this behaviour may also reflect components of natural appetitive behaviour and hence be used as an indicator of immediate postprandial satiety. This behaviour may also be performed in a stereotypic manner (e.g. bar-biting).
Apart from stereotypic behaviour and rooting or manipulative behaviour, physical activity may also reflect satiety. It has been shown that when feed is restricted there is an increase in activity(Reference Beattie and O'Connell 19 ), which corresponds with the observation that locomotion is an integral part of appetitive foraging strategies in pigs(Reference Stolba and Wood-Gush 5 ). Theoretically, in contrast with stereotypies, which appear to be mainly elicited by a meal(Reference Terlouw, Wiersma, Lawrence and MacLeod 17 ), physical activity and rooting or manipulative behaviour may be shown not only just after a meal, but also several hours later, anticipating the next meal. Thus, these behaviours may also reflect prolonged satiety.
Voluntary feed intake, and particularly time between subsequent feeding bouts, may also be a potential indicator of prolonged satiety. Data on voluntary feed intake patterns over the 24 h cycle, however, are often lacking in studies with dietary fibre. Thus, in the present paper, the focus will be on behaviours that potentially reflect satiety.
Potential influences of dietary fibre on satiety
Although extensively studied, there is still no consensus about the definition of dietary fibre. The physiological definition, ‘the dietary components resistant to degradation by mammalian enzymes’, was most commonly used in the past. Nowadays, the chemical definition ‘the sum of NSP and lignin’ is increasingly used. It has been suggested that non-digestible oligosaccharides and resistant starch (RS) may be added(Reference Champ, Langkilde, Brouns, Kettlitz and Le Bail-Collet 24 ); however, lignin was not mentioned. Fig. 1 shows the different components of dietary fibre and a subdivision of NSP with reference to the Van Soest analysis(Reference Van Soest, Robertson and Lewis 25 ). General aspects of dietary fibres will be briefly described as a basis for the evaluation of their potential effects on behaviour and satiety. A detailed description of their characteristics is available in a review(Reference Bach Knudsen 26 ).
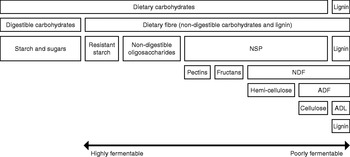
Fig. 1. Schematic representation of dietary carbohydrate, fibre and NSP composition with reference to the Van Soest analysis(Reference Van Soest, Robertson and Lewis 25 ). NDF, neutral-detergent fibre; ADF, acid-detergent fibre; ADL, acid-detergent lignin.
Plant fibres are generally components with relatively low energetic value, especially for omnivorous and carnivorous animals. The bulkiness properties of dietary fibre may, however, reduce feeding motivation by increasing mastication time and by stimulating mechanoreceptors in the gastrointestinal tract (sensory-specific satiety(Reference Paintal 27 , Reference Rolls and Rolls 28 )). This process could particularly stimulate satiation during a meal, promoting meal termination and thereby limiting meal size(Reference Cummings and Overduin 29 ). Fibre sources can be bulky as a result of their coarse structure (low bulk density, e.g. wheat-straw) or a high water-holding capacity (e.g. SBP). Soluble dietary fibres generally increase the viscosity of the lumen contents and therefore reduce gastric emptying rate and delay absorption of nutrients in the small intestine.
Dietary fibres can be partly fermented by intestinal microbiota, predominantly in the large intestine. Generally, soluble fibres are more completely and rapidly fermented than insoluble fibres. The resulting fermentation end products (SCFA, such as lactate, butyrate, propionate and acetate) can be used as energy sources. In growing pigs SCFA can contribute ⩽30% of the daily maintenance requirements for energy(Reference Bergman 30 , Reference Stevens and Hume 31 ). As SCFA (mainly acetate) become available in the distal part of the gut at times when glucose absorption is decreasing(Reference Bergman 30 , Reference Rérat 32 , Reference Van Leeuwen and Jansman 33 ), they may enhance satiety several hours after a meal. In sows an interprandial increase in physical activity has been observed, starting 5–6 h after a meal at a time that coincides with a drop in blood glucose levels(Reference de Leeuw, Jongbloed and Verstegen 34 ). In sows fed a high-fibre (SBP) diet this increase is lower and also interprandial blood glucose levels are more stable. Table 1 shows the relative estimated fermentability, water-holding capacity and bulk density of several fibre sources that are cited throughout the present paper.
Table 1. Comparison of some commonly-used sources of dietary fibre in pig research, based on fermentability, water-holding capacity (WHC) and bulk densityFootnote *

+, ++, +++, ++++, Increasingly higher extent of fermentability and WHC relative to the other fibre sources; −, −−, −−−, increasingly lower extent of bulk density relative to the other fibre sources.
* Estimation based on comparison of several literature sources(Reference Bauer, Williams, Voigt, Mosenthin and Verstegen 35 , Reference Robert, Matte, Farmer, Girard and Martineau 42 , Reference Auffret, Ralet, Guillon, Barry and Thibault 56 –Reference Tsaras, Kyriazakis and Emmans 59 ).
† A low bulk density indicates a high bulkiness.
Hypotheses
It is hypothesized that (1) bulky fibres (including fibres with a high water-holding capacity), irrespective of their fermentability, reduce mainly stereotypies, but also physical activity and substrate-directed rooting and manipulative behaviours in the first hours after a meal in pigs, indicating enhanced immediate postprandial satiety; (2) highly-fermentable fibres, irrespective of their bulkiness properties, reduce physical activity and substrate-directed behaviours for many hours after a meal, indicating a prolonged postprandial satiety. As stereotypies appear to be more closely related to the immediate postprandial period, prolonged effects on these behaviours are not expected.
Effects of dietary fibre in pigs
Reported effects of dietary fibres on immediate postprandial behaviour (i.e. within 3 h after a meal) and prolonged postprandial behaviour (i.e. >4 h after a meal) are shown in Tables 2 and 3 respectively. The studies are shown approximately in order of (in vitro) fermentability of the fibre sources used(Reference Bauer, Williams, Voigt, Mosenthin and Verstegen 35 ) and their percentage inclusion. The choice of behavioural categories is based on a study of the behavioural repertoire of group-housed pregnant sows that were assumed to be satiated (approximating to ad libitum intake of a high-fibre diet) in comparison with sows that were assumed not to be satiated (restrictedly fed a low-fibre diet)(Reference Zonderland, De Leeuw, Nolten and Spoolder 36 ). Self-directed behaviour includes stereotypies such as sham-chewing, tongue-playing and teeth-grinding, and substrate-directed behaviour includes behaviours that are directed at substrates such as the floor, the wall, bars and bedding material, when present (e.g. rooting, licking and biting) and are sometimes also performed in a stereotypic manner (e.g. chain-biting or bar-biting). The category general oral behaviour has been added because in some studies self-directed and substrate-directed behaviour have been measured as one category.
Table 2. Review of immediate postprandial effects of dietary fibre on behaviour in sows, measured directly after meals (i.e. during 0·75–3 h after a meal) in different studies

SBP, sugarbeet pulp; OTH, oat hulls; WB, wheat bran; GM, green-grass meal; PK, palm-kernel expeller; CP, citrus pulp; SH, soyabean hulls; near-AL, sows were allowed to feed ad libitum during a 30 min period; LE, low energy; BE, same diet as LE, but balanced for energy by adding fat; ↓, ↔, decreased and not affected by dietary treatment respectively.
* No. of meals per d.
† Feeding frequency was part of a 2×2 factorial design.
‡ Tendency.
§ Pre- and post-feeding behaviour were pooled.
Immediate postprandial effects
In all studies self-directed behaviour, substrate-directed behaviour, general oral behaviour and physical activity directly after feeding are either reduced or not influenced by the inclusion of dietary fibre in the diet of sows (Table 2). The effects are not dependent on the fermentability of the fibre source, as both poorly-fermentable (e.g. OTH) and highly-fermentable (e.g. SBP) fibres reduce abnormal behaviours and physical activity. As no fibre sources with a low bulkiness (e.g. RS) were included in the studies reported, no firm conclusions of the effect of bulkiness per se can be drawn.
Self-directed behaviour immediately after a meal is reduced by SBP(Reference Brouns, Edwards and English 11 , Reference Zonderland, De Leeuw, Nolten and Spoolder 36 ) and a mixture of fermentable fibre sources(Reference De Leeuw, Zonderland, Altena, Spoolder, Jongbloed and Verstegen 37 , Reference Van der Peet-Schwering, Spoolder, Kemp, Binnendijk, den Hartog and Verstegen 38 ), but not by OTH(Reference Bergeron, Bolduc, Ramonet, Meunier-Salaun and Robert 12 ) (Table 2). Self-directed behaviour was not measured in studies with wheat bran (WB). General oral behaviour (including self- and substrate-directed stereotypies), however, is reduced by both OTH(Reference Bergeron, Bolduc, Ramonet, Meunier-Salaun and Robert 12 , Reference Robert, Bergeron, Farmer and Meunier-Salaün 39 ) and WB(Reference Ramonet, Robert, Aumaître, Dourmad and Meunier-Salaün 18 ). WB is not as effective as SBP in reducing oral stereotypies(Reference Ramonet, Robert, Aumaître, Dourmad and Meunier-Salaün 18 ); however, this finding is only reported in an observation period that includes feeding, and when SBP is compared with WB and the control diet there is a higher feeding time, leaving less time for non-feeding activities.
All fibre sources tested reduce substrate-directed behaviour after feeding. Increasing levels of a mixture of fibre sources does not influence substrate-directed behaviour, but does linearly reduce total oral behaviour after a meal(Reference De Leeuw, Zonderland, Altena, Spoolder, Jongbloed and Verstegen 37 ) (data not shown in Table 2). This finding is in line with the hypothesis that postprandial oral behaviour may be a good indicator of the satiating effects of dietary fibres. In contrast, immediately-postprandial satiety is not always clearly reflected in general physical activity. For instance, in some studies SBP(Reference Brouns, Edwards and English 11 , Reference Zonderland, De Leeuw, Nolten and Spoolder 36 , Reference Braund, Edwards, Riddoch and Buckner 40 , Reference Danielsen and Vestergaard 41 ), a mixture of fibre sources(Reference Van der Peet-Schwering, Spoolder, Kemp, Binnendijk, den Hartog and Verstegen 38 ) and OTH(Reference Bergeron, Bolduc, Ramonet, Meunier-Salaun and Robert 12 , Reference Robert, Matte, Farmer, Girard and Martineau 42 ) reduce physical activity, whereas in other studies they do not(Reference Whittaker, Spoolder, Edwards, Lawrence and Corning 23 , Reference de Leeuw, Jongbloed and Verstegen 34 , Reference De Leeuw, Zonderland, Altena, Spoolder, Jongbloed and Verstegen 37 , Reference Ramonet, Meunier-Salaün and Dourmad 43 –Reference Whittaker, Edwards, Spoolder, Lawrence and Corning 45 ). WB does not affect immediately-postprandial physical activity, but it was only tested in two studies, in one of which OTH was not found to be effective(Reference Robert, Rushen and Farmer 44 ).
With the exception of two studies(Reference Brouns, Edwards and English 11 , Reference Ramonet, Robert, Aumaître, Dourmad and Meunier-Salaün 18 ), feeding time was excluded or corrected for when measuring oral behaviour directly after a meal. For physical activity it is not always clear whether authors made this correction. As dietary fibres prolong eating time(Reference Brouns, Edwards and English 11 , Reference Bergeron, Bolduc, Ramonet, Meunier-Salaun and Robert 12 , Reference Ramonet, Robert, Aumaître, Dourmad and Meunier-Salaün 18 , Reference Whittaker, Spoolder, Edwards, Lawrence and Corning 23 , Reference Robert, Bergeron, Farmer and Meunier-Salaün 39 , Reference Danielsen and Vestergaard 41 , Reference Ramonet, Meunier-Salaün and Dourmad 43 , Reference Robert, Rushen and Farmer 44 , Reference Holt, Johnston, Baidoo and Shurson 46 ) and sows feed in standing posture, postprandial physical activity in sows fed fibrous diets could be overestimated when feeding time is not taken into account (for example, see Ramonet et al. (Reference Ramonet, Meunier-Salaün and Dourmad 43 ) and Robert et al. (Reference Robert, Rushen and Farmer 44 )).
In growing pigs studies on behavioural effects of dietary fibres are lacking. Several studies, however, have measured the effects of dietary fibre on energy expenditure associated with physical activity. During the first hours after meal time activity-related heat production (AHP) is reduced in group-housed restrictedly-fed growing pigs, which has been observed for SBP-silage(Reference Schrama, Verstegen, Verboeket, Schutte and Haaksma 47 ) (Fig. 2) and RS(Reference Bolhuis, Van den Brand, Staals, Zandstra, Alferink, Heetkamp and Gerrits 48 , Reference Schrama and Bakker 49 ) (Fig. 3). Immediately-postprandial effects of RS(Reference Bolhuis, Van den Brand, Staals, Zandstra, Alferink, Heetkamp and Gerrits 48 , Reference Schrama and Bakker 49 ) cannot be attributed to its bulkiness (including water-holding capacity), which is low (Table 1) and does not differ from the bulkiness of the digestible starch used in the control diets, but are probably caused by other characteristics of RS. Unfortunately, effects of RS on behaviour have not been measured in sows.
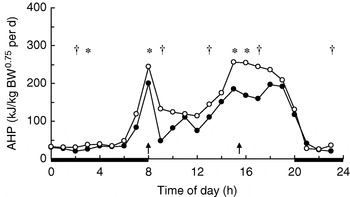
Fig. 2. Daily pattern of activity-related heat production
(AHP) in group-housed growing pigs fed a
high-starch diet (○) or a high-NSP
diet (•). BW, body
weight; ![]() , Feeding-time;
—, period of dark.
Mean values for the time of day were significantly different between
diets: *P<0·05, †P<0·10.
(From Schrama et
al. (Reference Schrama, Verstegen, Verboeket, Schutte and Haaksma
47
); reproduced with
permission of the Journal of Animal
Science.)
, Feeding-time;
—, period of dark.
Mean values for the time of day were significantly different between
diets: *P<0·05, †P<0·10.
(From Schrama et
al. (Reference Schrama, Verstegen, Verboeket, Schutte and Haaksma
47
); reproduced with
permission of the Journal of Animal
Science.)
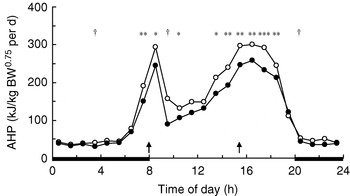
Fig. 3. Daily pattern of activity-related heat production
(AHP) in group-housed growing pigs fed a
pregelatinized maize starch (○) or
native potato starch (•) diet,
calculated from data of Schrama &
Bakker(Reference Schrama and Bakker
49
). BW, body weight;
![]() ,
Feeding-time; —, period of dark. Mean values for the time
of day were significantly different between diets:
*P<0·05,
**P<0·01,
***P<0·001, †P<0·10.
,
Feeding-time; —, period of dark. Mean values for the time
of day were significantly different between diets:
*P<0·05,
**P<0·01,
***P<0·001, †P<0·10.
Prolonged postprandial effects
Fermentable fibre sources clearly reduce physical activity for several hours after feeding, indicating prolonged postprandial satiety. The effects of poorly-fermentable sources such as OTH and WB on physical activity are less conclusive (Table 3). Self-directed behaviour was measured for a longer period post-feeding only in studies in which fermentable fibre sources were fed, and the levels shown are very low. In general, substrate-directed behaviour and total oral behaviour are reduced by both highly- and poorly-fermentable fibre sources.
Table 3. Review of prolonged postprandial effects of dietary fibre on behaviour in sows, measured during a preprandial (PP) or interprandial (IP; i.e. >4 h after a meal and not directly preceding a meal) period or over 12–24 h in different studies

SBP, sugarbeet pulp; SBPS, sugarbeet-pulp silage; OTH, oat hulls; WB, wheat bran; PK, palm-kernel expeller; CP, citrus pulp; SH, soyabean hulls; near-AL, sows were allowed to feed ad libitum during a 30 min period; LE, low energy; BE, same diet as LE, but balanced for energy by adding fat; PP1, before the first of two meals; PP2, before the second of two meals; ↑, ↓, ↔, increased, decreased and not affected by dietary treatment respectively.
* No. of meals per d.
† Feeding frequency was part of a 2×2 factorial design.
‡ Time period when lights were on.
§ Tendency.
As was expected, self-directed stereotypic behaviour is a poor indicator of prolonged postprandial satiety. Except for the immediate postprandial period (until 3 h after feeding), the levels of this behaviour are generally very low. SBP, however, is able to reduce self-directed behaviour before a meal to even lower levels, but only in one study in which sows that received the SBP-rich diet were allowed to eat large amounts(Reference Zonderland, De Leeuw, Nolten and Spoolder 36 ). General oral behaviour before a meal and over the 24 h cycle (including both self- and substrate-directed behaviours) is reduced by a mixture of fermentable fibre sources(Reference De Leeuw, Zonderland, Altena, Spoolder, Jongbloed and Verstegen 37 , Reference Ramonet, Meunier-Salaün and Dourmad 43 ) and by OTH(Reference Bergeron, Bolduc, Ramonet, Meunier-Salaun and Robert 12 ).
In contrast to the hypothesis, poorly-fermentable fibres from OTH and WB also reduce substrate-directed behaviour in the longer term(Reference Robert, Matte, Farmer, Girard and Martineau 42 , Reference Robert, Rushen and Farmer 44 ); it should be noted that only chain manipulation was observed and measurements took place mainly just before feeding time in these studies. It is possible that manipulation of the chain, which was located near the trough, may have been compensatory behaviour, anticipating the lack of satisfaction of chewing needs associated with the upcoming fibre-poor meal.
Physical activity is clearly reduced by fermentable fibre sources(Reference Ramonet, Robert, Aumaître, Dourmad and Meunier-Salaün 18 , Reference de Leeuw, Jongbloed and Verstegen 34 , Reference Zonderland, De Leeuw, Nolten and Spoolder 36 , Reference De Leeuw, Zonderland, Altena, Spoolder, Jongbloed and Verstegen 37 , Reference Braund, Edwards, Riddoch and Buckner 40 , Reference Ramonet, Meunier-Salaün and Dourmad 43 , Reference Whittaker, Edwards, Spoolder, Lawrence and Corning 45 , Reference McGlone and Fullwood 50 , Reference Rijnen, Verstegen, Heetkamp, Haaksma and Schrama 51 ); the only exception is a study in which time spent lying over the 24 h cycle is increased by soyabean hulls (see Holt et al. (Reference Holt, Johnston, Baidoo and Shurson 46 )). Physical activity over the 24 h cycle is reduced by the poorly-fermentable WB as effectively as SBP in one study(Reference Ramonet, Robert, Aumaître, Dourmad and Meunier-Salaün 18 ). However, WB has no effect on physical activity(Reference Robert, Matte, Farmer, Girard and Martineau 42 , Reference Robert, Rushen and Farmer 44 ), with even an increase in the frequency of posture changes before the second meal during the second pregnancy(Reference Robert, Matte, Farmer, Girard and Martineau 42 ). In three studies physical activity is reduced by OTH, although only during the 12 h light period in one study(Reference Bergeron, Bolduc, Ramonet, Meunier-Salaun and Robert 12 ) and not over 24 h in the other two studies(Reference Robert, Bergeron, Farmer and Meunier-Salaün 39 , Reference Robert, Matte, Farmer, Girard and Martineau 42 ). During the second pregnancy frequency of posture changes is affected by OTH, while standing is increased(Reference Robert, Matte, Farmer, Girard and Martineau 42 ) (not shown in Table 3). In another study physical activity is not affected by OTH(Reference Robert, Rushen and Farmer 44 ). Thus, results of the poorly-fermentable OTH and WB on physical activity over the 24 h cycle are not conclusive. Highly-fermentable fibres, therefore, have a higher potential to prolong postprandial satiety. This conclusion is supported by a study in which the daily voluntary intake of several high-fibre diets was measured, which shows that the ingestion of diets with SBP (40–65%, w/w) is much lower (2·3–5·0 kg/d) than that of diets with OTH (37%; 7·7 kg/d) or WB (67%; 7·1 kg/d) (Reference Brouns, Edwards and English 52 ). The SBP-rich diets thereby reduce voluntary feed intake, with digestible energy intake approximating to levels that are acceptable for optimal reproduction. A study of the effect of a diet containing 45% (w/w) SBP fed ad libitum to gestating sows on their performance has concluded that feeding a high level of fermentable NSP ad libitum during three successive reproduction cycles has no negative effects on reproductive performance(Reference van der Peet-Schwering, Kemp, Plagge, Vereijken, den Hartog, Spoolder and Verstegen 53 ).
Experimental evidence suggests that some (SBP, RS), but not all (soyabean hulls, solvent-extracted coconut meal), highly-fermentable fibre sources reduce AHP in group-housed growing pigs (Table 4). SBP silage(Reference Schrama, Verstegen, Verboeket, Schutte and Haaksma 47 ) (Fig. 2) and SBP(Reference Rijnen, Van den Borne, Schrama, Gerrits, Souffrant and Metges 54 ) tend to reduce AHP over the 24 h cycle in growing pigs, but the effect is predominantly present close to feeding. RS reduces AHP over the 24 h cycle, with the effects being significant not only after feeding, but also before the afternoon meal(Reference Bolhuis, Van den Brand, Staals, Zandstra, Alferink, Heetkamp and Gerrits 48 , Reference Schrama and Bakker 49 ) (Fig. 3). In one study all treatments increase AHP about 5 h before the second meal (i.e. 4 h after the first meal), with a decrease shortly after each meal(Reference Bolhuis, Van den Brand, Staals, Zandstra, Alferink, Heetkamp and Gerrits 48 ). This finding is in agreement with those of a study that reports an interprandial increase in physical activity in sows starting 6–7 h before the second meal (i.e. 5–6 h after the first meal)(Reference de Leeuw, Jongbloed and Verstegen 34 ). The highest AHP is shown by pigs fed the control diet and housed in straw-bedded pens(Reference Bolhuis, Van den Brand, Staals, Zandstra, Alferink, Heetkamp and Gerrits 48 ) (2×2 factorial design). The authors attribute this outcome to the presence of straw being an outlet for the increased motivation to display foraging behaviour in pigs fed the control diet. However, the addition of milled wheat straw to the diet, in order to increase its bulkiness, does not significantly reduce AHP over the 24 h cycle(Reference Schrama and Bakker 49 ). In sows on a high-fibre diet (SBP and soyabean hulls) AHP is reduced between meals, but not when expressed as a 24 h average, probably because of increased eating time of the high-fibre diet(Reference Ramonet, Van Milgen, Dourmad, Dubois, Meunier-Salaün and Noblet 55 ). SBP does not have a clear effect on AHP over the 24 h cycle, but a comparison of the two lowest fibre levels with the two highest fibre levels (contrast analysis), shows a decrease in AHP in the pigs on the highest-fibre diets(Reference Rijnen, Verstegen, Heetkamp, Haaksma and Schrama 51 ).
Table 4. Review of prolonged effects of dietary fibre on activity-related heat production (AHP) in studies with group-housed growing pigs (GP) and sows (S)Footnote *
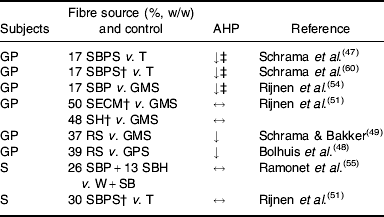
SBP, sugarbeet pulp; SBPS, sugarbeet-pulp silage; T, tapioca; GMS, gelatinized maize starch; SECM, solvent-extracted coconut meal; SH, soyabean hulls; RS, resistant starch; GPS, gelatinized potato starch; W+SB, wheat+soyabean meal; ↓, ↔, decreased and not affected by dietary treatment respectively.
* Animals were group-housed in all experiments, except Ramonet et al. (Reference Ramonet, Van Milgen, Dourmad, Dubois, Meunier-Salaün and Noblet 55 ). Feeding levels were 1·3 and 2·5×maintenance requirement for sows and growing pigs respectively, except Schrama et al. (Reference Schrama, Verstegen, Verboeket, Schutte and Haaksma 47 ) (2×maintenance requirement) and Ramonet et al. (Reference Ramonet, Van Milgen, Dourmad, Dubois, Meunier-Salaün and Noblet 55 ) (sows received 37·2 MJ digestible energy/d).
† Dose–response study, the highest inclusion level is given.
‡ Tendency.
Conclusion
As was hypothesized, stereotypies and substrate-directed behaviours are reduced by dietary fibre immediately following a meal, irrespective of its source, indicating enhanced immediately-postprandial satiety. These oral behaviours, therefore, appear to be useful for measuring immediately-postprandial effects of dietary fibre on satiety and feeding motivation. General physical activity after feeding appears to be a less-reliable indicator of immediately-postprandial satiety. The effects of dietary fibre on oral behaviours after feeding are most probably associated with bulkiness properties. The effects of resistant starch, low in bulk but highly fermentable, on postprandial behaviour and satiety in pigs remain to be studied.
Highly-fermentable dietary fibres clearly reduce physical activity for many hours after a meal, whereas the effects of poorly-fermentable fibre sources are less conclusive. This finding suggests that highly-fermentable fibres prolong satiety and reduce feeding motivation for many hours after feeding. Except for the immediate postprandial period, very low levels of stereotypies are generally shown. Thus, as expected, physical activity, but not stereotypic behaviour, appears to be a reliable indicator of prolonged postprandial satiety. There is no conclusive evidence of specific characteristics of fibre sources having prolonged effects on substrate-directed behaviours, as both highly- and poorly-fermentable fibres reduce this behaviour. However, the effects of poorly-fermentable fibre sources have only been measured in a few studies and mainly just before meals. It is not expected that they have prolonged effects on satiety and feeding motivation for many hours after a meal.
It has been demonstrated that the effects of dietary fibre on satiety and feeding motivation depend on specific characteristics of fibre sources used. Thus, when searching for diets that optimally stimulate long-term satiety in sows, but probably also in other animal species and in man, it is important to take these characteristics into account.
Acknowledgements
Each author contributed fully to the review presented in this manuscript. This work was fully funded by Wageningen University. The authors have no conflicts of interest to report.











