Abstract
Predation involves more than just predators consuming prey. Indirect effects, such as fear responses caused by predator presence, can have consequences for prey life history. Laboratory experiments have shown that some rodents can recognize fear in conspecifics via alarm pheromones. Individuals exposed to alarm pheromones can exhibit behavioural alterations that are similar to those displayed by predator-exposed individuals. Yet the ecological and evolutionary significance of alarm pheromones in wild mammals remains unclear. We investigated how alarm pheromones affect the behaviour and fitness of wild bank voles (Myodes glareolus) in outdoor enclosures. Specifically, we compared the effects of exposure of voles living in a natural environment to a second-hand fear cue, bedding material used by predator-exposed voles. Control animals were exposed to bedding used by voles with no predator experience. We found a ca. 50% increase in litter size in the group exposed to the predator cue. Furthermore, female voles were attracted to and males were repelled by trap-associated bedding that had been used by predator-exposed voles. Movement and foraging were not significantly affected by the treatment. Our results suggest that predation risk can exert population-level effects through alarm pheromones on prey individuals that did not encounter a direct predator cue.
Similar content being viewed by others
Introduction
In 1961, Robert Ardrey wrote, “sex is a sideshow in the world of the animal, for the dominant color of that world is fear”1. Of course, the purveyors of fear in the world of the animal are predators, and their ultimate threat comes in the form of killing and eating prey. However, predation involves more than just predators consuming prey2,3. In fact, when facing only the risk of predation, prey can exhibit an array of anti-predatory responses, which have arisen through co-evolution with predators4. Such awareness or perception of predators by prey can affect the physiology, body condition, and reproduction in many prey species (reviewed by Lima 19982). At the population level, the effects of fear of predation may be of the same magnitude or greater than the actual act of predation5. While predation fear responses (sometimes placed under the umbrella of “stress”) are often seen as negative or costly for the prey, these responses can be adaptive6. For example, fear of predation can result in physiological changes in pregnant females that prepare offspring for life under high predation pressure7.
Early predator detection is necessary for prey to maximize their own fitness8. In many vertebrate predator-prey systems, the key cue of predator presence is scent9. Prey can assess predation risk by eavesdropping on predator scent marks left for intraspecific communication. An adaptive prey response requires recognition and correct interpretation and response to the cue. While fresh cues may suggest predation is imminent and a change in behaviour is needed, old cues should be ignored10. Behavioural responses by prey to avoid predation can include reduced activity or escape to another, often lower-quality, habitat11,12. These responses can translate to changes in foraging behaviours, decreased body condition13, and ultimately reduced fitness. In females, poor body condition can diminish offspring quantity and quality14; in males, predator avoidance during the mating season may lead to missed mating opportunities15 or breeding delays. Hence, if one looks back at the world described by Andrey, indirect interactions between the sideshow of sex and the world of fear are evident.
Many organisms, including vertebrates, invertebrates, and plants, respond to predation threats by producing alarm signals. (reviewed by Verheggen et al.)16. For example, some rodents are known to produce alarm pheromones (AP), chemical cues that warn conspecifics about possible dangers17,18. Neurobiological and psychological experiments show that individuals can recognise stressed conspecifics from the AP left behind following a stressful situation19. The receivers of this indirect signal increase their vigilance and risk assessment behaviour, i.e., change their behaviour in ways that are similar to individuals that encountered a direct predator cue18. Furthermore, murine AP is biochemically related to predator-produced scent cues common to most carnivores20; both contain similar sulfur-containing volatiles21.
In general, a role for fear in ecology of animals is recognized22; however, the ecological and evolutionary significance of AP as a fear cue in wild mammals remains unclear. To the best of our knowledge, only two studies have investigated the role of AP in wild mammals. In an experimental indoor arena, Cabrera voles (Microtus cabrerae) avoided areas with the scent marks of conspecifics that experienced various predation risks23. These predation risks included handling (simulation of capture), audio playbacks of an avian predator, and visual contact with a mammalian predator. In the same study, but under field conditions, Cabrera voles tended to decrease their activity near sites treated with AP. In another study, black-tailed deer (Odocoileus hemionus columbianus) produced AP when disturbed or alarmed24. In the presence of AP, female black-tailed deer became more alert and left the site more often than in the presence of control odours, odourless air, or deer urine24. Most other AP studies have used lab-strain rodents and unnatural stimuli, such as electric foot-shocks17,18.
Wild rodents are ideally suited for studying the ecology of predator cues. These animals have a highly developed olfactory system, and odours play a key role in their behavioural decision-making25. We tested the hypothesis that wild bank voles (Myodes glareolus), a model prey species in many studies of predator-prey interactions, would show antipredator-like responses when exposed to AP11,26, since the murine AP shares structural similarity with predator scent21. More specifically, we made the following predictions. First, bank voles are expected to decrease movement in response to AP11. Second, voles are expected to be more fearful and thus forage less efficiently in response to AP15,27. Third, these behavioural and ecological responses should lead to poorer body condition, which in turn should lead to a smaller proportion of breeding females and a smaller litter sizes on average26,28.
Results
Litter size was significantly greater by 1.9 ± 0.7 pups in AP enclosures compared to control enclosures (p = 0.013; Table 1, Fig. 1). Previous pregnancy and the interaction between treatment and previous pregnancy were not significant. None of these three terms, which also comprised the pregnancy model, accounted significantly for variation in the proportion of pregnant females (Table 1). Treatment did not have a significant effect on the female survival (control = 55 ± 12%, treatment = 50 ± 10%; Table 1).
Litter size ± SE in the control treatment, which received bedding materials of non-predator-exposed male bank voles, and in the AP treatment, which received beddings materials of predator- exposed male voles. Sample size inside the bars. Letters denote statistically significant difference between bars.
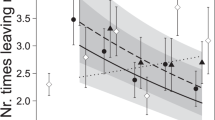
The interaction between treatment and sex significantly affected bait-less trapping (p = 0.019; Table 1, Fig. 2a). Females were trapped more and males less in the presence of AP. Neither treatment, sex, nor their interaction significantly affected trappability (Table 1). Only sex and the number of trapping instances significantly affected movement: females moved less than males (p = 0.012, females 70 ± 13 m2, males 177 ± 45 m2; Table 1, Fig. 2b) and more trapping instances correlated with greater movement areas (p < 0.001; 63.48 ± 11.33 m2 per trapping instance, Table 1).
GUD was significantly higher in areas where the vegetation was open compared to grass-covered areas (by on average 38.8 ± 3.8 seeds, p < 0.001; Table 1, Fig. 3). None of the other model terms accounted significantly for variation in GUD.
Giving up density (GUD) ± SE measured as number of seed left in the seed-trays in AP and control enclosures. All seed trays contained 80 sunflower seeds mixed with two liters of sand. White bars represent GUD boxes in dense vegetation; grey bars represent GUD boxes in open areas. Sample size inside the bars. Letters denote statistically significant difference between bars.
Discussion
Conspecific alarm cues impact fitness
In the current study, exposure to conspecific AP increased litter size by about 50% compared to control treatment. This result supports our hypothesis that voles can receive different information from predator-exposed and unexposed male conspecifics, and it suggests that female and male voles can respond differently to this information. Chemical cues can serve as an important means of communication among animals, both within and between species. Some rodents AP to warn conspecifics about possible dangers17,18,23. AP produced by mice are known to be molecularly similar to chemical cues produced by predators21. The observed effects of AP in voles may also be rooted in biochemical similarity, for example, to other compounds that signal danger natural settings, but this possibility requires further investigation.
Increased investment in reproduction by female bank voles faced with a predator cue is in line with several classic hypotheses concerning residual reproductive value29 and terminal investment30. We conducted the experiment late in the breeding season with the prediction that females should heavily invest resources into current reproduction. Such investment is expected because future reproduction opportunities are improbable for several interacting reasons. The most important are likely the approach of winter and poor overwinter survival of females that have already reproduced31, combined with high overall mortality risks from predation32. In comparison to AP females, females in the control treatment did not increase their litter size and were still prepared to produce one or two litters during ongoing breeding season. Reproduction should continue as long as there are sufficient resources for doing so; bank vole breeding season in our latitude usually lasts until September33. The litter size effect of AP could be mediated by males (e.g., via sperm quality), but examples of such mechanisms are not currently described in the literature. Simple physiological mechanisms could allow bank voles females to increase litter size: bank voles are induced ovulators34, meaning that females can produce more pups by mating more with one or several different males35. Many animals, including sticklebacks (Gasterosteus aculeatus)36 and some songbirds, have been found to increase investment in reproduction when predation risk is experimentally increased37. However, such increases are not universal. In some cases, cessation or delay of reproduction may result from high levels of predation. This phenomenon has been previously described in bank voles in laboratory studies38,39 and in studies with challenging ecological conditions (e.g., variable food availability and predation risk) during the first reproductive window after winter26. Reduced reproduction, however, is expected to be an unlikely alternative late in a breeding season. Quantifying fear effect on prey fitness has been criticized because it is difficult to assess the magnitude of experimenter-induced predator cues40. While ecological experiments can deviate from natural conditions in some way, we factored in vole biology as much as possible (e.g., by using naturally occurring sex ratios and population densities and by adding scent cues produced by a single unique male per enclosure41.
Males and females respond differently to scent cues
Chemical cues produced by prey faced with a predator might communicate more than just alarm. For example, male lab mice exposed to either a predator (cat urine) or a predator/competitor (rat urine) produce cues that make themselves more attractive to female mice42,43. The element of our study involving trapping without seeds as bait offered support for this idea: female voles were more attracted to traps in the AP enclosures, where those females were presented with bedding used by the male voles that had encountered a predator. In contrast, male voles avoided traps in the AP enclosures and were more frequently found in traps in the control enclosures. The fact that only male voles had been used to produce both types of bedding materials in our study may be useful in linking our results to possible mechanisms. One possible candidate attracting females to the bedding materials from predator-exposed AP males could be the major urinary protein (MUP). Mice are able to regulate the total amount of MUP in their urine during social contacts, and female mice advertise their reproductive state by varying the concentration of MUP during the oestrous cycle44. MUP can function as a pheromone and stimulate sexual attraction45 and oestrus in female mice46. The chemical can also promote aggressive behaviour in male mice47 and extend the longevity of olfactory cues that they leave behind48. One or more urine components (e.g., MUP), and not urine itself, most likely drive our observed differences. Control and AP bedding was collected after being used by a vole for about 48 hours, and since bank voles are assumed to urinate roughly constant rate, both control and AP bedding should have been thoroughly contaminated with urine and feces. Furthermore, prairie voles (M. ochrogaster) and woodland voles (M. pinetorum) urine mark at the same rate with and a without predator cue49.
If the bedding material used by predator-exposed male voles contained AP that resembled direct odour cues from predators themselves, then we predicted trappability would be higher under control conditions (i.e., unstressed male used bedding), since the bedding materials were distributed in the chimneys near the traps. However, neither the two groups nor the two sexes differed significantly in trappability when baited traps were in use. The absence of an experimental effect might be due to the highly attractive food rewards (i.e., energetically rich sunflower seeds) that were used as a bait in the traps. During the breeding season, voles, especially female ones, have high energy demands, which may be met (at least partially) by consuming the sunflower seed bait. Additionally, the voles in this study may have adjusted to the relatively constant presence of AP, which was present when the voles were added to the enclosure and regularly refreshed until the pregnant females were removed. Ultimately, a behavioural shift favouring the gain of food rewards over the risks of predation may have occurred; such an adjustment matches well with the risk allocation hypothesis50.
We also hypothesised that the addition of bedding materials from predator-exposed males would restrict the movement area of voles in the experimental enclosures51. Decreasing movement area should effectively decrease the risk of encountering and being killed by a predator4. However, we were unable to document an effect of our experimental treatment on movement. Our results contrast with those of an earlier experiment on Cabrera voles, which tended to decrease activity near AP patches23. In our enclosures, movement may have been suppressed by the avian predators to such an extent that voles were unable to further reduce their movement in response to our AP treatment52. Overall low trappability (around 50%) compared to previous summer experiments (see for example Haapakoski et al., 201353,54) supports this finding. Not surprisingly, males were moving more than females regardless of predation risk. Males need to move over several females territory in search of potential mates to increase their fitness55.
Perceived predation risk is expected to increase at the expense of foraging56. Thus, we expected a higher GUD in enclosures with bedding materials from the predator-exposed voles; however, we were unable to document differences in GUD between treatment and control enclosures. GUD was higher in open areas compared to more densely vegetated areas in our experimental enclosures, suggesting that the GUD method worked. With voles seeming to avoid the avian predation risk associated with foraging in open areas, this result resembles predator facilitation57. In fact, previous research suggests that field voles (M. agrestis) prefer open habitats when facing a mammalian predator (least weasel) and densely vegetated areas when facing an avian predator (kestrel falcon, Falco tinnunculus)52.
The pregnancy rate was similar in the experimental and control enclosures. Under experimentally increased predation risk in laboratory studies, bank voles have been observed to suppress breeding with females actively avoiding copulation38. Yet breeding suppression has never been reported during the peak of the breeding season in the field58, and the breeding suppression in the lab might be a laboratory artefact. Breeding suppression or avoidance might only occur when resources are limited26,59.
The survival of females did not differ between treatment and control groups. Previous studies of bank voles have not found an effect of predation risk on survival during summer58,60, but an inverse relationship between predation risk and survival in winter has been reported26. In that case, the assumption was that reduced survival was a consequence of reduced foraging. In our current study, foraging and movement were not significantly affected by the experimental treatment, which might explain the uniformity in survival.
Conclusions
The effects of predation are multifaceted and extend far beyond the death and consumption of prey. For example, we found that bedding materials used by predator-exposed male voles contained cues AP that could exert strong life-history effects in field settings. Specifically, indirect effects of predation risk, which here was communicated by the presence of the scent cues, translated to 1) increased litter size and 2) an interaction with sex that affected the attractiveness of traps. Thus, AP can impact the fitness and behaviour of animals. Continued study of these cues and others in both field and lab settings will contribute to a more nuanced understanding of interactions between predators and prey and between the sideshow of sex and the world of fear.
Material and Methods
Experimental setup
We conducted our experiment in twelve uncovered outdoor enclosures (0.25 ha per enclosure, see Haapakoski et al.26 for complete details) and in the laboratory of Konnevesi Research Station in central Finland (62°37′N, 26°20′E). Experimental status (AP vs. control, six enclosures each) was randomly assigned. In each enclosure, 25 multiple capture traps (Ugglan Special Nr. 2, Grahnab, Hillerstorp, Sweden) were arranged in a 5 × 5 grid with 10 m intervals between traps (Fig. 4). The traps were covered with bottomless metal chimneys (40 × 40 × 50 cm3) on which a metal lid was placed to protect voles in the traps from direct sunlight and rain.
Enclosures used in our experiment were 2500 m2, surrounded by a steel wall, and contained 25 traps that had a distance of 10 m between them. At four trapping locations (circles) in each enclosure, we placed GUD boxes, two of which had a non-altered dense vegetation surrounding the boxes and two had an mown open areas surrounding the boxes.
Study animals
The bank vole (Myodes glareolus) is a small common boreal microtine rodent. Vole populations cycle in Scandinavia, and specialist predators play a large role in driving these cycles61. One such predator is the least weasel (Mustela nivalis nivalis), which specializes in hunting small mammals. Due to its small size, the weasel is able to hunt in the burrows of voles, leaving few safe places for the voles to escape predation51. As a consequence of high predation pressure in the wild, antipredator behaviours and physiology are important in bank voles.
In the study area, the breeding season of bank voles usually extends from the end of April to September. During the breeding season, female bank voles are strictly territorial and need an exclusive territory to breed. The territories of breeding males usually overlap with the territories of several females62. Bank voles are easy to trap in the field and maintain and breed in laboratory, thereby making the species an ideal subject for the study of predation risk.
We used sexually mature bank voles that were either enclosure- or laboratory-born. These voles were first-generation descendants of wild bank voles that had been originally trapped in the Konnevesi region. Prior to the experiment, these voles were housed individually under standard conditions60. Voles grouped together in an outdoor enclosure were unrelated. Enclosure groups were balanced based on vole body size and number of females who had previously given birth.
Production of alarm pheromone and control bedding materials
To produce AP and control bedding materials, we used six pairs of bank vole brothers from litters produced by six different parental pairs. Each of these twelve male offspring was placed individually into a standard rodent cage containing 3 l of wood shavings. A randomly selected vole from each parental pair was used for AP production, and these six were housed in the same room. The six that produced the control bedding were housed in a separate room.
In order to induce AP production, we exposed the six AP voles to a weasel. For the first two weeks, a caged weasel was placed daily on top of the cage of each AP vole for 2 minutes. Weasel cage was equipped with solid bottom in order to prevent weasel urine and feces mixing in to AP bedding. After this procedure, we captured each AP vole with our hands and released it back into its cage to simulate predator capture. The AP production procedure was changed two weeks after the start of the experiment in order to minimize stress for the weasel. With this new method, a bank vole was first captured by hand from its cage and placed individually inside a small wire mesh cage, which was then placed inside the weasel’s cage for 2 minutes. Afterwards, each vole was released back into its original cage. The weasel used in this procedure was housed under standard conditions60 in a cage that was kept in a different building from all voles.
About 48 h old bedding material originating from one specific male vole (either AP or control, see below) was assigned to a specific outdoor enclosure for the duration of the experiment. In all enclosures, 0.1 l of bedding material was placed in each trap chimney and around the giving-up density tray (see below). Bedding material (AP or control) was placed at the start of the experiment and replaced every other day during the experiment.
The experiment started with the spreading AP and control bedding materials into the enclosures at the end of July 2015. On the same day bedding materials were added to an enclosure, four females were released to allow for habituation to the environment and its cue of indirect predation risk or not. Three days after adding the females, four males were released into each enclosure. In order to determine female survival, the proportion of pregnant females, parturition date, and litter size, females were trapped and moved to the laboratory around 18 days after initiation of the experiment.
Giving-up density
To measure the voles’ perception of predation risk in each enclosure, giving-up density (GUD56) was measured using four boxes (20 × 20 × 15 cm3 covered with a transparent lids) accessible via two 2 cm diameter entrances. Two GUD boxes were placed in open vegetation, and two were placed under the grass and were thus more protected from avian predators (see Fig. 1). Open vegetation areas were created by mowing the grass in a 1 m diameter area where the GUD boxes were placed. GUD boxes contained 80 sunflower seeds mixed with 2 L of sand. GUD, quantified here the as the number of seeds remaining, was recorded after two consecutive 24 hr periods, which started on the afternoon of day 4 and ending on the afternoon of day 6. If voles perceive that predation risk outweighs foraging gains, then they will give up searching for seeds, and GUD will be high. In situations where they feel safer, the voles will spend more time searching for the diminishing seeds, and GUD will be lower.
Movement and trappability
To quantify movement area, all traps were activated and regularly checked, and the location of any captured individual was recorded before release at the point of capture. Movement area trapping was conducted on days 10 (evening only), 11 and 12 (both morning and evening), and 13 (morning only). The first movement area trapping (day 10 evening) was conducted without bait (hereafter “bait-less trapping”) to measure the effect of AP treatment, since the food reward might have otherwise lured voles into traps. In bait-less trapping traps were activated immediately after distributing AP and control bedding materials in the chimneys. Bait-less trapping is possible since voles are using trap chimneys and traps as hiding places when traps are deactivated. Movement areas (100% convex polygons) were calculated from the trapping data with the program Ranges VI (Anatrack ltd. Wareham, UK). Movement area calculations excluded individuals that were caught only once. Trappability was calculated per vole as the number of captures divided by six (the number of baited trap checks). Bait-less trapping, used for the calculation of the movement areas but not for trappability. At the start of the intensive trapping period, two individuals were found dead in one trap in one enclosure; these individuals were replaced.
Statistical analysis
R version 3.2.263 with package lme464 was used to perform linear mixed effects analyses, which accounted for the eight voles per enclosure. We used log-likelihood ratio tests and χ2 statistics to evaluate statistical significance (α = 0.05). Litter size was analysed by using a model that included treatment (AP/control), previous pregnancy (yes/no) and the interaction between the two. Movement area, trappability and bait-less trapping were analysed by using a model that included treatment (AP/control), sex (male/female), and the interaction between the two. In addition, the number of captures per individual was used as a covariate in the movement area analysis to account for differences in movement area due trappability. Female survival and female pregnancy were analysed with generalized linear mixed models (GLMM) with binomial error distribution. In the analysis of survival, treatment was used as a fixed factor. The pregnancy model, like the litter size model, included as fixed treatment, previous pregnancy (yes/no) and the interaction between the two. GUD was analysed using repeated measures analyses because GUD was measured on two days. For GUD, models included treatment, time (day one/two), their interaction, vegetation (open/covered), and the minimum number of voles known to be alive in the enclosure. Enclosure identity was included as a random factor in all analyses. Residuals were visually inspected for deviations from homoscedasticity and normality. In the movement area analysis, data were cube root transformed to meet the assumptions. For unknown reasons, four voles in one control enclosure died during the movement area trapping. Therefore, we excluded this enclosure from all analyses of data from after the point of mortality. (Data from this enclosure were used in analyses of GUD and bait-less trapping, which were collected before the mortality event.) We also excluded two individuals (IDs 4885 and 4886) in an AP treatment enclosure from our movement analyses, since their tag numbers were indistinguishable in the field.
Ethics
All work was conducted according Finnish legislation with the animal experimentation permission from Jyväskylä University No. ESAVI/6370/04.10.07/2014.
Data Accessibility
Data will be deposited to Dryad Digital Repository after acceptance.
References
Ardrey, R. African Genesis: A Personal Investigation Into the Animal Origins and Nature of Man (Book). (Atheneum, 1963).
Lima, S. L. Nonlethal Effects in the Ecology of Predator-Prey Interactions. Bioscience 48, 25–34 (1998).
MacLeod, K. J., Krebs, C. J., Boonstra, R. & Sheriff, M. J. Fear and lethality in snowshoe hares: the deadly effects of non-consumptive predation risk. Oikos 127, 375–380 (2018).
Lima, S. L. & Dill, L. M. Behavioral decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 68, 619–640 (1990).
Preisser, E. L., Bolnick, D. I. & Benard, M. F. Scared to death? The effects of intimidation and consumption in predator-prey interactions. Ecology 86, 501–509 (2005).
Boonstra, R. Reality as the leading cause of stress: rethinking the impact of chronic stress in nature. Funct. Ecol. 27, 11–23 (2013).
Sheriff, M. J., Krebs, C. J. & Boonstra, R. The sensitive hare: Sublethal effects of predator stress on reproduction in snowshoe hares. J. Anim. Ecol. 78, 1249–1258 (2009).
Ferrari, M. C. O., Wisenden, B. D. & Chivers, D. P. Chemical ecology of predator–prey interactions in aquatic ecosystems: a review and prospectusThe present review is one in the special series of reviews on animal–plant interactions. Can. J. Zool. 88, 698–724 (2010).
Apfelbach, R., Blanchard, C. D., Blanchard, R. J., Hayes, R. A. & McGregor, I. S. The effects of predator odors in mammalian prey species: a review of field and laboratory studies. Neurosci. Biobehav. Rev. 29, 1123–44 (2005).
Bytheway, J. P., Carthey, A. J. R. & Banks, P. B. Risk vs. reward: How predators and prey respond to aging olfactory cues. Behav. Ecol. Sociobiol. 67, 715–725 (2013).
Sundell, J. & Ylönen, H. Behaviour and choice of refuge by voles under predation risk. Behav. Ecol. Sociobiol. 56, 263–269 (2004).
Mäkeläinen, S., Trebatická, L., Sundell, J. & Ylönen, H. Different escape tactics of two vole species affect the success of the hunting predator, the least weasel. Behav. Ecol. Sociobiol. 68, 31–40 (2014).
Creel, S. & Christianson, D. Relationships between direct predation and risk effects. Trends Ecol. Evol. 23, 194–201 (2008).
Lindström, J. Early development and fitness in birds and mammals. Trends in Ecology and Evolution 14, 343–348 (1999).
Brown, J. S. Patch use as an indicator of habitat preference, predation risk, and competition. Behav. Ecol. Sociobiol. 22, 37–47 (1988).
Verheggen, F. J., Haubruge, E. & Mescher, M. C. Alarm pheromones-chemical signaling in response to danger. Vitam. Horm. 83, 215–39 (2010).
Zalaquett, C. & Thiessen, D. The effects of odors from stressed mice on conspecific behavior. Physiol. Behav. 50, 221–227 (1991).
Kiyokawa, Y., Kodama, Y., Kubota, T., Takeuchi, Y. & Mori, Y. Alarm pheromone is detected by the vomeronasal organ in male rats. Chem. Senses 38, 661–668 (2013).
Rottman, S. J. & Snowdown, C. T. Demonstration and analysis of an alarm pheromone in mice. J. Comp. Physiol. Psychol. 81, 483–490 (1972).
Ferrero, D. M. et al. Detection and avoidance of a carnivore odor by prey. Proc. Natl. Acad. Sci. USA 108, 11235–40 (2011).
Brechbühl, J. & Moine, F. Mouse alarm pheromone shares structural similarity with predator scents. Proc. R. Soc. B Biol. Sci. 112, 1–6 (2013).
Boissy, A. Fear and Fearfulness in Animals. The Quarterly Review of Biology 70, 165–191 (1995).
Gomes, L. A. P., Salgado, P. M. P., Barata, E. N. & Mira, A. P. P. Alarm scent-marking during predatory attempts in the Cabrera vole (Microtus cabrerae Thomas, 1906). Ecol. Res. 28, 335–343 (2013).
Müller-Schwarze, D., Altieri, R. & Porter, N. Alert odor from skin gland in deer. J. Chem. Ecol. 10, 1707–1729 (1984).
Carthey, A. J. R. & Banks, P. B. Naïveté in novel ecological interactions: Lessons from theory and experimental evidence. Biol. Rev. 89, 932–949 (2014).
Haapakoski, M., Sundell, J., Ylönen, H., Ylo, H. & Ylönen, H. Predation risk and food: opposite effects on overwintering survival and onset of breeding in a boreal rodent. J. Anim. Ecol. 81, 1183–92 (2012).
Bedoya-Perez, M. A., Carthey, A. J. R., Mella, V. S. A., McArthur, C. & Banks, P. B. A practical guide to avoid giving up on giving-up densities. Behav. Ecol. Sociobiol. 67, 1541–1553 (2013).
Korpimäki, E., Norrdahl, K. & Valkama, J. Reproductive investment under fluctuating predation risk: Microtine rodents and small mustelids. Evol. Ecol. 8, 357–368 (1994).
Williams, G. C. Natural Selection, the Costs of Reproduction, and a Refinement of Lack’s Principle. Am. Nat. 100, 687–690 (1966).
Clutton-Brock, T. H. Reproductive Effort and Terminal Investment in Iteroparous Animals. Am. Nat. 123, 212–229 (1984).
Prévot-Julliard, A. C., Henttonen, H., Yoccoz, N. G. & Stenseth, N. C. Delayed maturation in female bank voles: Optimal decision or social constraint? J. Anim. Ecol. 68, 684–697 (1999).
Tkadlec, E. & Zejda, J. Small rodent population fluctuations: The effects of age structure and seasonality. Evol. Ecol. 12, 191 (1998).
Eccard, J. A., Klemme, I., Horne, T. J. & Ylönen, H. Effects of competition and season on survival and maturation of young bank vole females. Evol. Ecol. 16, 85–99 (2002).
Clarke, J. R., Clulow, F. V. & Grieg, F. Ovulation in the bank vole, Clethrionomys glareolus. J. Reprod. Fertil. 23, 531 (1970).
Mokkonen, M., Koskela, E., Mappes, T. & Mills, S. C. Sexual antagonism for testosterone maintains multiple mating behaviour. J. Anim. Ecol. 81, 277–283 (2012).
Candolin, U. Reproduction under predation risk and the trade-off between current and future reproduction in the threespine stickleback. Proc. R. Soc. B Biol. Sci. 265, 1171–1175 (1998).
Mönkkönen, M., Forsman, J. T., Kananoja, T. & Ylönen, H. Indirect cues of nest predation risk and avian reproductive decisions. Biol. Lett. 5, 176–8 (2009).
Ylönen, H. & Ronkainen, H. Breeding suppression in the bank vole as antipredatory adaptation in a predictable environment. Evol. Ecol. 8, 658–666 (1994).
Ylönen, H. et al. Small mustelids and breeding suppression of cyclic microtines: adaptation or general sensitivity? Ann. Zool. Fennici 32, 171–174 (1995).
Peers, M. J. L. et al. Quantifying fear effects on prey demography in nature. Ecology 99, 1716–1723 (2018).
Norrdahl, K. & Korpimäki, E. Changes in individual quality during a 3-year population cycle of voles. 239–249, https://doi.org/10.1007/s004420100795 (2002).
Lixing, J. Z. Æ., Kevin, S. Æ. & Novotny, M. V. Chronic exposure of cat odor enhances aggression, urinary attractiveness and sex pheromones of mice. J. Ethol. 279–286, https://doi.org/10.1007/s10164-007-0060-1 (2008).
Liu, Y.-J. et al. Chronic Co-species Housing Mice and Rats Increased the Competitiveness of Male Mice. Chem. Senses 42, 247–257 (2017).
Stopka, P., Janotova, K. & Heyrovsky, D. The advertisement role of major urinary proteins in mice. Physiol. Behav. 91, 667–670 (2007).
Roberts, S. A. et al. Darcin: a male pheromone that stimulates female memory and sexual attraction to an individual male’s odour. BMC Biol. 8, 75 (2010).
Marchlewska-Koj, A., Cavaggioni, A., Mucignat-Caretta, C. & Olejniczak, P. Stimulation of Estrus in Female Mice by Male Urinary Proteins. J. Chem. Ecol. 26, 2355–2366 (2000).
Chamero, P. et al. Identification of protein pheromones that promote aggressive behaviour. Nature 450, 899–902 (2007).
Hurst, J. L., Robertson, D. H. L., Tolladay, U. & Beynon, R. J. Proteins in urine scent marks of male house mice extend the longevity of olfactory signals. Anim. Behav. 55, 1289–1297 (1998).
Wolff, J. O. Scent marking by voles in response to predation risk: a field-laboratory validation. Behav. Ecol. 15, 286–289 (2004).
Lima, S. L. & Bednekoff, P. A. Temporal Variation in Danger Drives Antipredator Behavior: The Predation Risk Allocation Hypothesis. Am. Nat. 153, 649–659 (1999).
Norrdahl, K. & Korpimäki, E. Does Mobility or Sex of Voles Affect Risk of Predation by Mammalian Predators? Ecology 79, 226–232 (1998).
Korpimäki, E., Koivunen, V. & Hakkarainen, H. Microhabitat use and behavior of voles under weasel and raptor predation risk: predator facilitation? Behav. Ecol. (1996).
Haapakoski, M., Sundell, J. & Ylönen, H. Mammalian predator-prey interaction in a fragmented landscape: Weasels and voles. Oecologia 173, 1227–1235 (2013).
Haapakoski, M., Lensu, A., Sundell, J., Vihervaara, H. & Ylönen, H. Infanticide effects on behavior of the bank vole (Myodes glareolus) in the fragmented breeding habitat. Behav. Ecol. Sociobiol. 69, 49–59 (2015).
Koskela, E., Mappes, T. & Ylonen, H. Territorial Behaviour and Reproductive Success of Bank Vole Clethrionomys glareolus Females. J. Anim. Ecol. 66, 341–349 (1997).
Brown, J. S. Vigilance, patch use and habitat selection: Foraging under predation risk. Evol. Ecol. Res. 1, 49–71 (1999).
Kotler, B., Blaustein, L. & Brown, J. Predator facilitation: the combined effect of snakes and owls on the foraging behavior of gerbils. Annales Zoologici Fennici 47, 465–468 (1992).
Trebatická, L., Suortti, P., Sundell, J. & Ylönen, H. Predation risk and reproduction in the bank vole. Wildl. Res. (2012).
Jochym, M. & Halle, S. To breed, or not to breed? Predation risk induces breeding suppression in common voles. Oecologia 170, 943–953 (2012).
Haapakoski, M., Sundell, J. & Ylönen, H. Conservation implications of change in antipredator behavior in fragmented habitat: Boreal rodent, the bank vole, as an experimental model. Biol. Conserv. 184 (2015).
Hanski, I., Henttonen, H., Korpimäki, E., Oksanen, L. & Turchin, P. Small Rodent Dynamics and Predation. Ecology 82, 1505–1520 (2001).
Bujalska, G. The role of spacing behavior among females in the regulation of reproduction in the bank vole. J. Reprod. Fertil. Suppl. 19, 465–74 (1973).
R Development Core Team, R. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing 1, 409 (2011).
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting Linear Mixed-Effects Models using lme4. (2014).
Acknowledgements
We thank the mammalian ecology and life histories research group lead by Prof. Hannu Ylönen for providing all of the facilities for our study and commenting the manuscript. Technicians at the Konnevesi Research Station did an excellent job of maintaining the enclosures. Alexandra Thiel’s help in the lab and in the field was valuable. Ines Klemme provided comment to the manuscript. Financial support came from the Jenny and Antti Wihuri Foundation (to M.H.). A.H. and K.D.M. thank the staff of the Resource Ecology Group for their support.
Author information
Authors and Affiliations
Contributions
M.H. and A.A.H. conceived the study; A.A.H. and M.H. carried out field experiment; M.H., K.D. M. and A. A. H. performed statistical analyses; and M.H., K.D.M. and A.A.H. wrote the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Haapakoski, M., Hardenbol, A.A. & Matson, K.D. Exposure to Chemical Cues from Predator-Exposed Conspecifics Increases Reproduction in a Wild Rodent. Sci Rep 8, 17214 (2018). https://doi.org/10.1038/s41598-018-35568-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-35568-0
Keywords
This article is cited by
-
Conspecific disturbance odors act as alarm cues to affect female mate choice in a treefrog
Behavioral Ecology and Sociobiology (2022)
-
Sexual differences in responses of meadow voles to environmental cues in the presence of mink odor
Animal Cognition (2022)
-
Bank vole alarm pheromone chemistry and effects in the field
Oecologia (2021)
-
Effect of early postnatal exposure to 17α-ethinylestradiol on female rat reproductive physiology
Toxicology and Environmental Health Sciences (2021)
-
Effects of predator-induced stress during pregnancy on reproductive output and offspring quality in Brandt’s voles (Lasiopodomys brandtii)
European Journal of Wildlife Research (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.