Abstract
Oxygen limitation and dietary imbalances are key aspects influencing feed intake (FI) and growth performance in cultured fish. This study investigated the combined effects of hypoxia and dietary electrolyte balance on the growth performance, body composition and nutrient utilization in a rainbow trout (Oncorhynchus mykiss) isogenic line. Fish were fed ad libitum two experimental diets: electrolyte-balanced or -imbalanced diets (DEB 200 or 700 mEq kg−1, respectively) and exposed to normoxia or hypoxia (7.9 or 4.5 mg O2 l−1, respectively) for 42 days. DEB did not affect FI, growth performance or body composition. Nevertheless, hypoxia had a negative impact, reducing FI (6%), growth rate (8%), oxygen consumption (19%), energy (5%) and lipid (42%) contents. Digestible energy intake and heat production were higher in normoxic fish (40% and 23%, respectively), retaining 64% more energy in lipid or protein. Hypoxia reduced the apparent digestibility of dry matter, ash, protein, lipid, carbohydrates and energy. Trout fed DEB 700 diet were energetically less efficient, reflected in higher heat production and energy requirements for maintenance. FI was inhibited by low dissolved oxygen levels, but not by electrolyte-imbalanced diet, in spite of the higher energy requirements for maintenance. This study highlights the importance that dietary-electrolyte content and DO levels have on energy balance and growth performance when fish are fed to satiation.
Similar content being viewed by others
Introduction
Feed intake (FI) is the main determinant of animal growth. In fish, voluntary FI is influenced by dietary, environmental and/or physiological factors. The impact of dissolved oxygen (DO) level on FI has been widely documented in different fish species1,2,3,4, including the rainbow trout5,6. In general, FI decreases with decreasing DO, as this physiological trait is limited by the oxygen uptake capacity7. At normoxia and in the absence of other constraints, the long term (weeks) FI of fish can be constrained by a set-point value of oxygen consumption. It is proposed that diet composition may affect the amount of oxygen consumed per unit of feed and this may posse further limits in fish subjected to hypoxia8. However, marked intraspecific variation in the metabolic phenotype of fishes has been described, which are suggested to be influenced by both genes and developmental conditions9.
Fish may display metabolic depression as part of an adaptive response to a stress situation10 (e.g. hypoxia), resulting in downregulation of metabolic activity by multiple signalling factors at tissue and cellular levels11. European sea bass (Dicentrarchus labrax) chronically exposed to low DO and crowding have been shown to decrease their FI and energy requirements for maintenance12. Similarly, energy requirements for maintenance in Nile tilapia (Oreochromis niloticus) were reduced when exposed to low DO levels4. In particular, it has been suggested that energy requirements for maintenance could be lessened in rainbow trout (Oncorhynchus mykiss) exposed to hypoxia5, implying that nutrient utilization could be modulated by DO levels. Thus, fish exposed to sub-optimal conditions, including low DO, reduce their FI and partially compensate this with more efficient nutrient utilization. Nevertheless, the combined effects of hypoxia and diet composition on dietary nutrient utilization are still poorly understood.
Saravanan et al.13 showed that both hypoxia (DO 4.0 mg O2 l−1) and an amino-acid-imbalance diet produced a significant reduction in FI in rainbow trout. However, the study showed that oxygen consumption in trout were unaffected by the dietary imbalance. Similarly, the oxygen consumption in Nile tilapia were not altered by dietary lipid to carbohydrate ratios, while FI was affected14. Both studies suggest that nutritional factors affecting energy use and oxygen availability could pose constraints on FI, although fish may have effective mechanisms to compensate for changes in energy intake in order to achieve energy balance.
The acid-base homeostasis (pH) is one of the most important physiological processes in fish, while dietary and environmental conditions may deeply alter this balance15. Acid-base homeostasis disturbances appear to increase the oxygen consumption required for maintenance metabolism in fish, as the animal needs several energy consuming processes essential to keeping this balance. In fact, the oxygen consumption rate increases in rainbow trout reared at pH either below or above the optimal pH16. Regulation of systemic pH is then achieved by adjusting the rates of acid and/or base excretion, which in turn are linked to ion uptake through the involvement of ions exchange mechanism occurring mainly in the gill17,18,19. The kidney plays a complementary role in acid-base balance by reabsorbing HCO3− from the filtrate18. In addition, the enzyme carbonic anhydrase present in gill and kidney is involved in the regulation of acid-base balance of freshwater rainbow trout20.
In fish, the acid-base homeostasis can be affected by the dietary electrolyte balance (DEB) which in turn will alter the maintenance energy expenditure21,22. The DEB is defined as the sum of the mineral cations minus the sum of mineral anions present in the diet. Differences in DEB may occur when feed ingredients containing different quantities of cations (Na, K, Ca and Mg) and anions (Cl and P) are included in the diet formulation23. A low DEB diet has acidic properties, while a high DEB diet has alkaline properties. Alterations in DEB can trigger mechanisms to counteract acid-base imbalances. The gastro-intestinal tract (GIT) and gills produce acid-base secretions for the reestablishment of the acid-base homeostasis, at the expense of an extra energetic cost. Previous studies in Nile tilapia and meagre fed either 200 DEB or electrolyte- imbalanced diets (800 and 700 DEB, respectively) have shown that maintenance energy expenditure increases with DEB level24,25. However, studies on the impact of DEB on growth, FI and nutrient digestibility in fish have been limited, in spite of the overlapping roles of the GIT in water/ionic balance and nutrient digestion/assimilation. Feeding fish with different DEB may enable us to elucidate the limits of the oxygen consumption, energy use and availability which are required for growth, having important practical implications for fish production.
The current study investigated the changes in FI on growth performance, oxygen consumption, nutrient digestibility, nitrogen and energy balance, together with several metabolic markers in an isogenic heterozygous family of rainbow trout (Oncorhynchus mykiss) subjected to a combination of nutritional (DEB 200 or DEB 700) and environmental (normoxia or hypoxia) challenges. An isogenic trout line was employed in this study for their genetic uniformity, providing an experimental model with low intra-specific variability, thus high reproducibility.
Results
Feed intake, growth performance and oxygen consumption
Figure 1 presents FI expressed per unit of metabolic body weight (FIMBW), growth rate expressed by metabolic body weight (GRMBW), hepato-somatic index (HSI), feed conversion ratio (FCR), oxygen consumption (OC) and the coefficient between oxygen consumption and growth rate (OC/GRMBW). FIMBW, GRMBW, OC or OC/GRMBW were not affected by the diet, but were altered by DO levels (P < 0.01) (Table 1). FIMBW (6.7%) and GRMBW (1.2%) were higher in normoxic when compared to hypoxic groups. Also, OC and the relation between OC and GRMBW were significantly higher in normoxia than in hypoxia (24% and 14%, respectively). On the other hand, HIS and FCR were not altered by DO level, but were 16% and 4.6% respectively higher in fish fed the DEB 700 diet (P < 0.05). No interactions between DEB and DO levels were observed for these parameters. No mortalities were recorded during the trial, except in the DEB 200 treatment in hypoxic condition (98.9% survival).
Effects of dietary electrolyte balance (DEB) and dissolved oxygen levels on (A) feed intake expressed per unit of metabolic body weight (FIMBW); (B) growth rate expressed in metabolic body weight (GRMBW); (C) hepatosomatic index (HSI); (D) feed conversion ratio (FCR); (E) oxygen consumption (OC) and (F) oxygen consumption to growth rate (OC/GRMBW) of rainbow trout. Further details are provided in Methods. Values are mean ± SEM (n = 3 tanks, except for HSI where n = 9 fish). Different letters indicate differences among treatments (α = 0.050).
Body composition
Dietary treatment did not affect body composition, while low DO levels decreased dry matter, lipids and energy (P < 0.05) (Table 2). Normoxic fish had 9% more lipids and about 4.5% more energy than hypoxic fish. The DEB and DO interaction did not significantly affect body nutrient (proteins, lipids) and energy content.
Nutrient digestibility, nitrogen and energy balances
The apparent nutrient and energy digestibility coefficients are presented in Table 3 and are used to calculate the nitrogen and energy balance parameters presented in Tables 4 and 5, respectively. DEB affected the digestibility of dry matter (P < 0.001), ash (P < 0.001), lipid (P < 0.001) and energy (P < 0.05), but did not affect protein and carbohydrate digestibility coefficients. DO level affected the digestibility of dry matter (P < 0.001), ash (P < 0.05), proteins (P < 0.001), lipids (<0.01), carbohydrates (P < 0.001) and energy (P < 0.01). However, no interaction between the DEB and DO levels was detected.
DEB did not affect the nitrogen balance (Table 4), although protein efficiency (PE) was higher in trout fed the DEB 200 diet (P < 0.05). In addition, DO level affected gross nitrogen (P < 0.01, GN) and digestible nitrogen intake (P < 0.01, DN), branchial and urinary nitrogen loss (P < 0.01, BUN) and retained nitrogen (P < 0.05, RN), but did not affect PE. Normoxic fish had higher DN (41%) and RN (42%) than hypoxia groups.
DEB affected heat production (P < 0.01) and metabolizable energy for maintenance (P < 0.001) (Table 5). Heat production and energy requirements for maintenance were 26% and 65% higher, respectively, in DEB 700 diets than in DEB 200 diets. On the other hand, DO levels affected all energy balance parameters (P < 0.01), with the exception of maintenance. Gross energy (GE) and digestible energy intake (DE) were 43% and 40% higher respectively in the normoxic fish. Metabolizable energy (ME), heat production (HP) and retained energy (RE) were also 40%, 23% and 54%, respectively, higher in the normoxic fish. The DO levels affected energy retention in the form of lipids (P < 0.01) and protein (P < 0.05). Normoxic group retained more energy as lipid (64%) and protein (42%) than hypoxic group.
Changes in blood and chyme parameters
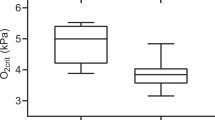
The effects of DEB and DO levels on the O2-carrying capacity at 2 and 6-h post-prandial are presented in Fig. 2, and the statistical analyses are presented in Table 6. Dietary treatment has no effect on HCT, Hb or MCHC. An interaction between the diet and DO levels for MCHC was observed (P < 0.05). Hypoxic fish fed the DEB 700 diet had lower SSI and WBCV than fish fed the DEB 200 diet (P < 0.05) 6-h after feeding. HCT were higher (P < 0.001) and WBCV lower (P < 0.001) in the hypoxic fish than in the normoxic fish, regardless of the dietary treatment. The effect on WBCV was more pronounced in fish fed the DEB 700 diet, at 6-h after feeding (P < 0.05), and an interaction between diet and DO levels was observed (P < 0.05).
Effects of dietary electrolyte balance (DEB) and dissolved oxygen levels on (A) hematocrit (HCT); (B) haemoglobin (Hb); (C) mean corpuscular haemoglobin concentration (MCHC); (D) white-blood cell volume (WBCV) and (E) spleno-somatic index (SSI) of rainbow trout 2 or 6 h after feeding. Further details are provided in Methods. Values are mean ± SEM (n = 9). Different letters indicate differences among treatments, while the asterisk indicates differences between sampling times (α = 0.050).
The post-prandial effects of DEB and DO levels on blood pH and chyme characteristics in trout are presented in Fig. 3 and the statistical analysis in Table 7. Diet had a clear effect on blood pH, both in the heart and the caudal region. The pH was significantly lower in heart (P < 0.01) and the caudal region (P < 0.05) in fish fed the diet with alkaline properties (DEB 700). Conversely, the chyme pH was significantly higher in fish fed the DEB 700 diet (P < 0.001). The chyme dry matter was lower in fish fed DEB 700 (P < 0.001) when compared with DEB 200 (Fig. 3). DO levels had a significant effect on blood pH. Fish subjected to hypoxic conditions had a higher blood pH values when measured in the heart (P < 0.05) and in the caudal region (P < 0.001). However, chyme pH and DM were not affected by DO levels. Stomach chyme pH was significantly affected by the interaction between diet post-prandial time (P < 0.001). Blood pH, stomach chyme DM and pH decreased over time after feeding (P < 0.001).
Effects of dietary electrolyte balance (DEB) and dissolved oxygen levels on (A) heart blood pH; (B) caudal blood pH; (C) chyme pH, and (D) dry matter content in the stomach of rainbow trout 2 or 6 h after feeding. Further details are provided in Methods. Values are mean ± SEM (n = 9). Different letters indicate differences among treatments, while the asterisk indicates differences in sampling times (α = 0.050).
Discussion
The current study investigated the metabolic response of an isogenic rainbow trout line (R23) chronically exposed to a combination of nutritional (electrolyte-imbalanced diet, DEB 700 mEq Kg−1) and environmental (hypoxia, 4.5 ± 0.1 mg O2 l−1) stressors, by analysing changes on FI, growth performance, nutrient digestibility and energy balance. Feeding rainbow trout an electrolyte-balanced diet (DEB 200) caused lower metabolizable energy requirements for maintenance (MEm) when compared with fish fed the electrolyte-imbalanced diet (DEB 700), without significant changes in FI itself, growth performance, or oxygen consumption. The increase of MEm in the DEB 700 group may indicate an increase (65%) in the energy usage to maintain acid-base balance. A comparable increase in MEm values (54%) was observed in Nile tilapia fed the DEB 800 diet, when compared to fish fed the DEB 200 diet24. In contrast to the current study, Saravanan et al.24 detected that feeding a DEB 800 diet to Nile tilapia for 35 days caused a 15% decrease in growth rate.
In spite of an increase in the energy use in trout fed the DEB 700 diet, FI remained similar in both dietary treatments. This was surprising, as higher FI was expected to occur in trout fed the DEB 200 diet. This assumption was based on an expected higher O2 availability (larger scope for growth) in trout consuming the DEB 200 diet, as an increased O2 demand will be anticipated in fish fed electrolyte-imbalanced diet (DEB 700) to keep the fish’ homeostasis. As previously observed in trout, a decrease in FI with the increase in the dietary energy (lipid) content, may reflect a reduced scope for growth. Thus, factors affecting O2 availability may determine the limits in FI8,26 (a.k.a. oxystatic concept for FI). Therefore, results from this study do not correspond with the oxystatic concept. Nevertheless, the lack of differences in FI may be related to differences in diet composition, such as a higher palatability due to increased Na2CO3/Diamol content in the DEB 700 diet. Although no noticeable differences were observed in the feeding behaviour of trout fed DEB 200 or DEB 700 diets, future experiments should further investigate the effects of dietary electrolyte composition on diet palatability.
As expected, normoxic fish displayed higher oxygen consumption than the hypoxic group, indicating limited oxygen availability, which constrains FI in trout. Hypoxia was reflected in a 35% decrease in growth and FI when compared to the normoxic group. Results are consistent with previous reports showing decreased FI due to reduced O2 availability in rainbow trout5,6. On the other hand, feed conversion ratio (FCR) and efficiency in the utilization of dietary digestible protein (PE) were not affected by DO levels, confirming the previous observations in rainbow trout5. However, Glencross et al.5 have shown larger decreases in growth (54%) and FI (46%) in hypoxic trout (5.7 ± 1.4 mg O2 l−1) compared to the group in normoxia (9.3 ± 0.4 mg O2 l−1). Such differences may be due to the use of smaller trout (approximately half the size) by Glencross et al. compared to this study, rather than a differential response of the isogenic trout line to low DO levels. It has been suggested that low DO levels could have a stronger impact in bigger fish, posing a more strict limitation on FI and growth due to the allometric relationship between the gill surface area and BW27.
Feeding trout the electrolyte-imbalanced diet (DEB 700) did not result in changes in oxygen consumption, as the value for this parameter remained similar between both dietary groups (2.4 and 2.0 mg O2 kg−0.8 min−1 in normoxia and hypoxia, respectively). These results are in line with a previous study by Saravanan et al.13 in which oxygen consumption was identical in trout fed amino-acid-balance or -imbalance diets under normoxic (2.8 mg O2 kg−0.8 min−1) or hypoxic (2.4 mg O2 kg−0.8 min−1) conditions. Greater oxygen consumption measured by Saravanan et al.13 for both environmental DO levels may be due to the use of smaller trout than in our study (half BW), as similar conditions in the aquatic metabolic unit were implemented in both studies regarding the contrast in DO between hypoxia and normoxia.
Results showed that trout in normoxia displayed higher values for all the nitrogen (GN, DN, BUN, RN) and energy balance parameters (GE, DE, BUE, ME, RE, HP) than in hypoxia. However, normoxic fish showed lower values for all the digestibility parameters analysed (protein, carbohydrates, lipids and energy) than in the hypoxic group. These results suggested that rainbow trout was able to increase the efficiency of nutrient digestibility when chronically exposed to low DO levels. However, protein utilization in trout remained similar under different DO levels, which suggest an increased reliance on carbohydrates, and lipids to less degree, as energy sources under hypoxic conditions. Similarly, Glencross et al.5 found that protein utilization remained similar in trout under hypoxia. They suggested that hypoxic trout may increase the energy efficiency by decreasing the energy allocated toward lipid synthesis, as lipid metabolism is more sensitive to oxygen availability as an energy source. We propose that hypoxic trout may be able to partially compensate the decrease in energy availability, as a consequence of reduced FI, by increasing the nutrient digestibility for all macronutrients. Such compensation may also involve an increased efficiency of metabolic pathways related with carbohydrate and lipid metabolism during hypoxia. The proposed increases in both digestibility and metabolic energy efficiencies in fish exposed to chronic hypoxia requires further investigation to distinguish their potential contribution toward achieving energy balance.
Fish liver has a central role supplying energy to the tissues, with a remarkable capacity to store and mobilize energy reserves in response to several environmental and nutritional cues28. In particular, the hepatosomatic index (HSI) is a useful tool as a means of appraising metabolic capacity. For example, low levels of the essential amino acid lysine in experimental diets were reflected in higher HSI in rainbow trout29. In our study, the HSI of trout increased by 32% in normoxia, and 10% in hypoxia, when fed the alkaline diet. These changes suggest that trout fed the DEB 700 diet displayed a different metabolic capacity at a hepatic level, probably reflecting the increased energy demand necessary to maintain acid-base balance.
Regarding effects of the diet on nutrient digestibility, our study showed that apparent digestibility of DM and ash in trout fed the DEB 700 diet was higher than in the DEB 200 group. This positive effect on nutrient digestibility could be attributed to an increased fluid secretion into the stomach, with higher food liquefaction and lower chyme pH in the intestine of fish fed the alkaline diet, as has been previously hypothesized in the Nile tilapia by Saravanan et al.24. This interpretation is reinforced by the lower chyme DM content in the stomach of trout 2 and 6 hs after being fed the DEB 700 diet when compared to the DEB 200 group.
Higher Diamol content in the DEB 200 diet may have reflected in larger ash content of the faeces in fish fed this diet compared to the DEB 700 group. If ash digestibility is corrected for Diamol addition, assuming it to be fully inert, the ADC for ash is 42.9 and 54.0% for the DEB 200 and DEB 700 diets, respectively. The difference in ash ADC might furthermore be caused by leaching of Na2CO3 from the feed prior to consumption of the pellet. However complete leaching of Na2CO3 was not very likely because fish were consuming at least 75% of the feed very quickly and also due to the presence effects DEB on post prandial blood pH.
Previous studies have shown that there is a very large addition of fluid to the chyme in the stomach of trout, which continues as the digestion proceeds by endogenous water addition and/or secretion of digestive fluids in the gastro-intestinal tract of fish30,31,32. Regarding the mechanisms that may be involved, it has been previously suggested a higher liquefaction of the chyme in the stomach of fish fed high DEB levels can be due to altered osmolality, promoting the hydration of the content with endogenous water by osmosis, and/or increased secretion of digestive fluids24. Liquefaction of a dry feed in the stomach may produce a feed consistency resembling the natural feed, which may facilitate the digestive processes, as the chyme DM content is inversely related to nutrient digestibility32.
Apparent digestibility for phosphorus, lipid and energy were lower in fish fed the alkaline diet (DEB 700). This is surprising, as a previous study in Nile tilapia showed that increasing DEB levels had a positive effect on the digestibility of nutrients, which could partially compensate the increased maintenance requirements induced by highly alkaline diets24. In contrast to our study, Saravanan et al. showed that lipid digestibility was unaffected by DEB levels. Differences in the digestibility between both studies may be entirely due to the different feeding method employed, as tilapia were fed restrictively24, whereas our trout were fed to apparent satiation. However, the changes detected in trout’s digestibility could be due to a higher DM content of the DEB 700 as well, which may require higher liquefaction, lowering the intestinal chyme pH and decreasing the efficiency of digestive lipases. Also differences in chyme characteristics between both diet groups (e.g. stomach pH; Fig. 3) could be responsible for the observed differences in the phosphorus digestibility.
The isogenic trout line responded to long term low DO levels by boosting their O2-carrying capacities, mainly by increasing the hematocrit values. Results are in agreement with previous studies showing an enhancement on the O2-carrying capacity of rainbow trout under environmental hypoxia33,34. Splenic contraction and subsequent erythrocyte release into the circulation has been shown to occur in fish after acute hypoxia35. A previous study in rainbow trout has shown that the spleen remained contracted (lower SSI) when exposed to hypoxia (approximately 3.23 mg O2 l−1 DO), although SSI values return to normal after 6 days36. In our study, the SSI in trout was not significantly different between normoxic and hypoxic conditions when fed the DEB 200. However, lower SSI values were found in hypoxic trout 6 hs after being fed the DEB 700 diet. Decreased SSI values may be explained by a reduction on the haemoglobin’s O2-carrying capacity due to altered acid-base balance in fish fed the alkaline diet, which will require in counterpart an enhancement of this capacity under hypoxia by the release of erythrocyte from the spleen under hypoxia.
The results of our study showed that nutritional (DEB diets) and environmental (DO levels) factors produced changes in gastric chyme and blood pHs. As expected, the pH of the stomach chyme was higher in fish fed the alkaline diet (DEB 700), changes that were conspicuous even 6 hs after feeding. Alteration of chyme pH was reflected in a lower blood pH in the heart and the caudal region as well, as the fish digested the alkaline diet. Previous studies have shown that the rise in pH and HCO3− in arterial blood of trout after feeding (a.k.a. alkaline tide) is compensated by the excretion of base equivalents to the environment30,37. Similar results to our study have been observed in Nile tilapia 7 hs after feeding a DEB 800 diet, showing that blood pH in the heart was lower than in fish fed a DEB 200 diet24. This response suggests that the prolonged effects of a DEB diet on the systemic acid-base homeostasis of fish triggers mechanisms to fully compensate for the alteration in acid-base balance38,39. Therefore, rainbow trout when fed an alkaline diet appears to respond with a reduced alkaline tide, suggesting that the compensatory mechanisms taking place in the gills and kidney were more effective in this group than in fish fed the DEB 200 diet.
In the trout isogenic line, the pH of the blood was significantly increased in response to chronic hypoxic conditions. This contrasts with the blood acidification described by Claireaux et al.40 in trout exposed to acute hypoxia, with a subsequent increase in the haemoglobin affinity for O2. Internal alkalization of erythrocytes at the onset of acute hypoxia was believed to be responsible for the extracellular acidosis observed by Claireaux et al.40 in trout. However, blood acidification during hypoxia occurred when fish were fasted at least 24 h before measurements, contrasting with our study in which blood parameters in trout were analysed 2 or 6 h after feeding. Differences may due to the effect of digestion on blood pH combined with exposure to chronic hypoxia, as different adaptive mechanisms of the erythrocytes may take place when fasted trout is exposed to acute hypoxia. An interaction between DEB and DO levels was expected to occur in the FI and growth of trout, mainly due to the reduced O2-carrying capacity of haemoglobin in the DEB 700 group, but these effects were not observed in this study. This may be explained by the presence of compensatory mechanisms to the O2-binding properties of haemoglobin in response to stressors in rainbow trout41. Various forms of stressors have been shown to reduce the numbers and functions of white blood cells in fish42. We found decreases in the white blood-cell volume (WBCV) of trout exposed to hypoxia or when fed the DEB 700 diet. These observed decreases in WBCV were probably linked to higher hematocrit values detected in trout subjected to these treatments, although the mechanisms mediating both responses remain to be investigated.
In conclusion, FI was not affected by DEB in rainbow trout, but was decreased by long-term hypoxic conditions. DEB affected acid-base balance, as an electrolyte-imbalanced diet (DEB 700) required an increased energy expenditure to maintain acid-base homeostasis, although oxygen consumption remained unaffected in trout. Stomach chyme pH was higher in trout fed the alkaline diet (DEB 700), reflecting in lower blood pH in the heart and the caudal region as the digestion proceeded. Fish fed the DEB 700 responded with a reduced alkaline tide, suggesting that compensatory mechanisms were effective in reducing the impact triggered by the acid-base imbalance. As a result, feeding trout an electrolyte imbalanced diet resulted in significantly higher requirements for metabolizable energy for maintenance (MEm) than a diet with a better electrolyte balance (DEB 200). However, increased energy expenditure used towards acid-base regulation caused by the DEB 700 diet did not alter growth. This could be due to slight differences in FI, undetected in the present study, which may have compensated for a higher MEm of the DEB 700 diet.
This study shows the importance that dietary electrolyte balance (e.g. mineral content) and DO levels have on energy balance and growth performance when fish are fed to satiation. Understanding the factors affecting FI may assist to improve management and practical conditions of fish farming, for example by adjusting feed composition or rearing conditions. This is highly relevant as fish oxygen carrying capacity under intensive aquaculture systems may be changed by the use of novel feed ingredients and different rearing conditions, which ultimately impact FI.
Methods
Fish and housing
An isogenic heterozygous family of rainbow trout (R23), produced by crossing two homozygote isogenetic lines (GABI/La Peima, INRA, France)43, was used in this study. Fish were housed in the Aquatic Metabolic Unit (AMU) tanks of Aquaculture and Fisheries group, Wageningen University, The Netherlands. The tanks were connected to a common water recirculation system consisting of a trickling filter, an oxygenation unit, a sump, a drum filter (Hydrotech 500®) and a cooling/heating system for maintaining uniform water quality throughout the study. The oxygenation unit maintained the DO levels by injecting oxygen into the water, and was facilitated with separate automatic probes for the detection of water flow and oxygen consumption and was also equipped with faecal collectors for measuring digestibility as described by Saravanan et al.14. Water temperature was set at 14 ± 1 °C. Photoperiod was maintained at 12:12 (Light: Dark) with daybreak set at 07:00 h.
Experimental design
Rainbow trout were housed in the AMU, according to a 2 × 2 factorial design, with diet (DEB 200 or DEB 700) and water DO levels (normoxia or hypoxia) as factors. Twelve experimental tanks (200 L each) were divided into three blocks of four tanks in each block, and the four treatments were assigned randomly within each of three blocks (N = 3 tanks treatment−1). Three hundred and seventy fish were weighed (115.2 ± 2.0 g) and 30 fish were randomly assigned to each of the 12 tanks. Ten fish were randomly sampled for initial body composition analyses.
Experimental diets and feeding
Two isoproteic (45% DM) and isoenergetic (22 kJ gDM−1) diets (Table 8) were extruded by Research Diet Services (Wijk bij Duurstede, The Netherlands). The diet was a floating 4 mm pellet and contained 0.01% yttrium oxide as inert marker for the determination of the apparent digestibility coefficient. The two diets were formulated to provide a contrast in electrolyte content (DEB); 200 or 700 mEq Kg−1. This difference was created by adding different amounts of Na2CO3 and diamol (inert filler) in the diets.
Fish were fed the experimental diets to apparent satiation, twice a day for 42 days. At the end of each feeding session, feed given and uneaten feed pellets were counted to determine FI on a daily basis. Faeces were collected to determine nutrient digestibility in a similar manner as described by Amirkolaie et al.32.
Experimental conditions
Experimental tanks, connected to a common water recirculation system, had an oxygenation unit supplying water at a constant DO level (10.2 ± 0.1 mg O2 l−1) by injecting pure oxygen into the common inlet, which was regulated by a mass flow controller (Brooksw Model 5850 S; Brooks Instruments) and a microprocessor (Brooksw Read Out and Control Electronics Model 0154; Brooks Instruments). Each metabolic tank was equipped with a water flow meter (MAGFLOWw MAG 5000; Danfoss A/S) to regulate and monitor water flow (inlet). The volume of water within the tanks was kept identical (200 litres) by adjusting a standpipe. The water surface of each tank was covered with a water-resistant floating panel to prevent gas exchange between water and air. Within the floating panel, a circular feeding hatch (18·5 cm in diameter) with a removable floating lid was used to feed the fish.
The outlet of each metabolic tank was linked to a measuring hub to continuously measure DO (WTW-Trioximaticw 700 IQ; WTW GmbH), pH (WTW-SensoLyt DWw (SEA) 700 IQ; WTW GmbH) and conductivity (WTW TetraCon325w 700 IQ; WTW GmbH) of water. Measured values of DO, pH and conductivity were automatically recorded in a personal computer.
The difference in DO levels was induced by adjusting the rate of water inflow to the tanks as described by Saravanan et al.13. On this procedure, in the normoxic groups, the rate of water inflow to each tank was kept at 7.2 ± 0.0 l min−1 (mean ± SEM) with a mean water DO level of 10.2 ± 0.1 mg O2 l−1. The DO level in the outflowing water remained at 7.9 ± 0.1 mg O2 l−1. Hypoxia conditions were created by gradually reducing the rate of water inflow to the tank to reach a water flow of 2.2 ± 0.0 l min−1 of oxygenated water (10.2 ± 0.1 mg O2 l−1) for the first three days after the start of the experiment. After that time, the DO level in the outflowing water for each hypoxic tank was maintained at 4.5 ± 0.1 mg O2 l−1 until the end of the trial.
Critical oxygen tension for rainbow trout (Pcrit) has been reported to be 2.9 kPa at 15 °C44, 3 times lower than the O2 level used in this study (4.5 ± 0.1 mg O2 l−1 or 9.1 kPa at 14 °C). However, the DO level applied in the hypoxia treatment is recognized as an environmental challenge, with the value decided based on the reported critical DO level of 6.0 mg O2 l−1 for feed consumption and 7 mg O2 l−1 for both growth rate and feed conversion efficiency rainbow trout at 15 °C6.
The oxygen consumption of fish was monitored throughout the entire experimental period. Oxygen content in the inlet and outlet of each tank was automatically measured at 5 min intervals using an electrode (WTW-TrioximaticH 700 IQ, WTW GmbH, Weilheim, Germany) and data was recorded in a personal computer using an interface (HTBasic, Version 9.5, TransEra Corp.). The oxygen electrode was calibrated once a week.
Fish sampling
Fish were sampled at the start and at the end of the trial to determine initial (n = 10) and final body composition (n = 10 per tank), respectively. At 42 days of trial, fish were sampled at 2 h and 6 h after the morning feeding. Prior to sampling, fish were anesthetized with an overdose of 2-phenoxy ethanol (ml l−1) and then blood was drawn from the heart and caudal blood vessels with a heparinized syringe. Blood pH was immediately measured (pH meter, WTW pH 320; pH electrode, WTW SenTix Sp). The duration of anaesthesia (2 min) and the fish-handling period were strictly standardized for all fish to minimize blood pH variation24. After pH measurement, blood samples were used to measure hematocrit (HCT), white blood cell volume (WBCV) values, and haemoglobin (Hb) concentration. Fish were weighed and euthanized to sample gastric chyme, liver and spleen. Samples were frozen in liquid nitrogen and stored at −80 °C for later analyses.
Chemical Analysis
Whole fish from each tank (3 fish tank−1) were ground, pooled and fresh moisture content was determined. Fish and faeces were subsequently freeze-dried before further analyses. Feed, faeces and whole-body samples were analysed in triplicates for dry matter (105 °C for 24 h) and protein (Kjeldahl; N ∙ 6.25) after acid digestion. Lipid content of feed and faeces were analysed as described by Folch et al.45 and by petroleum ether extraction (Soxhlet; 40–60 °C) in the whole fish. Gross energy content was analysed in an adiabatic bomb calorimeter (IKA-Werke C5000). Ash content was determined by combustion in muffle furnace (550 °C for 12 h). The same ash samples of feed and faeces were used to determine acid insoluble ash46. Yttrium content in feed and faeces was measured by inductively coupled plasma atomic emission spectroscopy (ICP-AES)47.
Measurements and calculations
The percentage of fish survival (S) was calculated as:
where Nf is the final number of fish and Ni is the initial number of fish.
Mean metabolic weight of fish (MBWg) was calculated as:
where Wg is the geometric mean body weight (g).
FI per unit of mean metabolic body weight (FIMBW) was calculated as:
where FIABS is the daily absolute FI, calculated as:
where FITOT is the total FI per tank (g DM) over the experimental period corrected for dead fish and uneaten feed, n is the number of fish per tank and t is the experimental period (days).
Growth rate on metabolic weight (GRMBW) was calculated as:
where Wf is the mean final BW (g), Wi is the mean initial BW (g) and t is the duration of the growth study (days).
Feed conversion ratio (FCR) was calculated as:
The hepato-somatic index was calculated as:
The spleen-somatic-index was calculated as:
Mean corpuscular haemoglobin concentration was calculated as follows:
where [Hb] is the concentration of haemoglobin in blood (g dl−1) and Hct the hematocrit value (%).
Total carbohydrate content in diets and faeces were calculated as:
The apparent digestibility coefficients (ADC) of protein, lipid, total carbohydrates and energy were calculated by comparing the amount of yttrium (Y) as an inert marker in relation to the content of the nutrient in the feed and faeces, according to:
where X represents dry matter, crude protein, crude lipid, total carbohydrate, gross energy or ash, Ydiet and Yfaeces are the yttrium content in the diet and faeces, respectively, and Xdiet and Xfaeces are the X content in diet and faeces, respectively.
The gross nitrogen intake (GN) was calculated as:
The digestible nitrogen intake (DN) was calculated as:
The retained N (RN, mg N fish−1 day) was calculated as the difference between the N content of the final fish carcass and that of the initial fish carcass. Branchial and urinary N loss (BUN) was calculated as the difference between DN and RN.
Protein efficiency (PE) was calculated as:
Oxygen consumption of the fish was calculated per tank with the difference in measured concentration of oxygen between inlet and outlet, and the rate of water flow in the tank using the formula specified by Saravanan et al.14.
The parameters of energy balance (kJ fish−1 day) were calculated as follows: gross energy intake (GE) as the product of FI (g DM fish−1 day) and energy content of the diet; digestible energy intake (DE) as the product of GE and ADCE; metabolizable energy intake (ME) as the difference between DE and branchial and urinary energy loss (BUE), which was estimated as (BUN × 24.85)/1000, where 24.85 is the amount of energy (kJ) equivalent to 1 g excreted N, assuming that all N is excreted as NH3-N; retained energy (RE) as the difference between the energy content of the final fish carcass and that of the initial fish carcass. Heat production (HP, kJ kg−0.8 day) was calculated as the difference between ME and RE. Metabolizable energy for maintenance (MEm) was estimated as ME-(RE/0.65), where 0.65 is the efficiency of energy utilization for growth48. MEm was divided by Wg (kJ kg−0.8 day).
Use of experimental animals
The fish trials were approved and carried out according to the Wageningen University Ethics Board for experimentation with animals (DEC, Registration protocol 2014056.a), under Dutch and EU legislation on the handling of experimental animals.
Data availability statement format guidelines
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Foss, A., Evensen, T. H. & Oiestad, V. Effects of hypoxia and hyperoxia on growth and food conversion efficiency in the spotted wolffish Anarhichas minor (Olafsen). Aquaculture Research 33, 437–444, https://doi.org/10.1046/j.1365-2109.2002.00693.x (2002).
Pichavant, K. et al. Effects of hypoxia on growth and metabolism of juvenile turbot. Aquaculture 188, 103–114, https://doi.org/10.1016/S0044-8486(00)00316-1 (2000).
Thetmeyer, H., Waller, U., Black, K. D., Inselmann, S. & Rosenthal, H. Growth of european sea bass (Dicentrarchus labrax L.) under hypoxic and oscillating oxygen conditions. Aquaculture 174, 355–367, https://doi.org/10.1016/S0044-8486(99)00028-9 (1999).
Tran-Duy, A., van Dam, A. A. & Schrama, J. W. Feed intake, growth and metabolism of Nile tilapia (Oreochromis niloticus) in relation to dissolved oxygen concentration. Aquaculture Research 43, 730–744, https://doi.org/10.1111/j.1365-2109.2011.02882.x (2012).
Glencross, B. D. Reduced water oxygen levels affect maximal feed intake, but not protein or energy utilization efficiency of rainbow trout (Oncorhynchus mykiss). Aquaculture Nutrition 15, 1–8, https://doi.org/10.1111/j.1365-2095.2007.00562.x (2009).
Pedersen, C. L. Energy budgets for juvenile rainbow trout at various oxygen concentrations. Aquaculture 62, 289–298, https://doi.org/10.1016/0044-8486(87)90171-2 (1987).
Pauly, D. In Excellence in Ecology Vol. 22, 1–247 (2010).
Saravanan, S. et al. Voluntary feed intake in rainbow trout is regulated by diet-induced differences in oxygen use. Journal of Nutrition 143, 781–787, https://doi.org/10.3945/jn.112.173062 (2013).
Metcalfe, N. B., Van Leeuwen, T. E. & Killen, S. S. Does individual variation in metabolic phenotype predict fish behaviour and performance? Journal of Fish Biology 88, 298–321, https://doi.org/10.1111/jfb.12699 (2016).
Bonga, S. E. W. The stress response in fish. Physiological Reviews 77, 591–625, https://doi.org/10.1152/physrev.1997.77.3.591 (1997).
Richards, J. G. In Fish Physiology Vol. 27 (eds Richards, J. G. Anthony P. Farrell & Brauner, C. J.) Ch. 10, 443–485 (Academic Press, 2009).
Santos, G. A., Schrama, J. W., Mamauag, R. E. P., Rombout, J. H. W. M. & Verreth, J. A. J. Chronic stress impairs performance, energy metabolism and welfare indicators in European seabass (Dicentrarchus labrax): The combined effects of fish crowding and water quality deterioration. Aquaculture 299, 73–80, https://doi.org/10.1016/j.aquaculture.2009.11.018 (2010).
Saravanan, S. et al. Oxygen Consumption Constrains Food Intake in Fish Fed Diets Varying in Essential Amino Acid Composition. PLoS ONE 8, https://doi.org/10.1371/journal.pone.0072757 (2013).
Saravanan, S. et al. Control of voluntary feed intake in fish: a role for dietary oxygen demand in Nile tilapia (Oreochromis niloticus) fed diets with different macronutrient profiles. British Journal of Nutrition 108, 1519–1529, https://doi.org/10.1017/S0007114511006842 (2012).
Janssen, R. G. & Randall, D. J. The effects of changes in pH and PCO2 in blood and water on breathing in rainbow trout, Salmo gairdneri. Respiration physiology 25, 235–245 (1975).
Hargis, J. R. Ventilation and metabolic rate of young rainbow trout (Salmo gairdneri) exposed to sublethal environmental pH. The Journal of experimental zoology 196, 39–44, https://doi.org/10.1002/jez.1401960105 (1976).
Claiborne, J. B., Edwards, S. L. & Morrison-Shetlar, A. I. Acid-base regulation in fishes: Cellular and molecular mechanisms. Journal of Experimental Zoology 293, 302–319, https://doi.org/10.1002/jez.10125 (2002).
Perry, S. F. et al. Channels, pumps, and exchangers in the gill and kidney of freshwater fishes: Their role in ionic and acid-base regulation. Journal of Experimental Zoology Part A: Comparative Experimental Biology 300A, 53–62, https://doi.org/10.1002/jez.a.10309 (2003).
Evans, D. H., Piermarini, P. M. & Choe, K. P. The Multifunctional Fish Gill: Dominant Site of Gas Exchange, Osmoregulation, Acid-Base Regulation, and Excretion of Nitrogenous Waste. Physiological Reviews 85, 97–177, https://doi.org/10.1152/physrev.00050.2003 (2005).
Gilmour, K. M. & Perry, S. F. Carbonic anhydrase and acid–base regulation in fish. Journal of Experimental Biology 212, 1647–1661, https://doi.org/10.1242/jeb.029181 (2009).
Dersjant-Li, Y. et al. Metabolic costs of changing the cation-anion difference in the diet of juvenile African catfish Clarias gariepinus (Burchell). Aquaculture Nutrition 6, 39–45 (2000).
Dersjant-Li, Y. et al. The influence of dietary cation-anion differences on acid-base balance, food intake, growth and nutrient utilisation of juvenile African catfish Clarias gariepinus (Burchell). Fish Physiology and Biochemistry 20, 305–311 (1999).
Patience, J. F. & Wolynetz, M. S. Influence of Dietary Undetermined Anion on Acid-Base Status and Performance in Pigs. The Journal of Nutrition 120, 579–587 (1990).
Saravanan, S. et al. Dietary electrolyte balance affects the nutrient digestibility and maintenance energy expenditure of Nile tilapia. British Journal of Nutrition 110, 1948–1957, https://doi.org/10.1017/S0007114513001323 (2013).
Magnoni, L. J. et al. Dietary electrolyte balance affects growth performance, amylase activity and metabolic response in the meagre (Argyrosomus regius). Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, https://doi.org/10.1016/j.cbpb.2017.03.001.
Saravanan, S. et al. Constraints on energy intake in fish: The link between diet composition, energy metabolism, and energy intake in rainbow trout. PLoS ONE 7, https://doi.org/10.1371/journal.pone.0034743 (2012).
Tran-Duy, A., Schrama, J. W., van Dam, A. A. & Verreth, J. A. J. Effects of oxygen concentration and body weight on maximum feed intake, growth and hematological parameters of Nile tilapia, Oreochromis niloticus. Aquaculture 275, 152–162, https://doi.org/10.1016/j.aquaculture.2007.12.024 (2008).
Brusle, J. & Anadon, G. In Fish morphology (eds Dutta, H. M. & Datta-Mushi, J. S.) 434 (CRC Press, 1996).
Walton, M. J., Cowey, C. B. & Adron, J. W. The effect of dietary lysine levels on growth and metabolism of rainbow trout (Salmo gairdneri). British Journal of Nutrition 52, 115–122, https://doi.org/10.1079/BJN19840077 (1984).
Bucking, C. & Wood, C. M. Water dynamics in the digestive tract of the freshwater rainbow trout during the processing of a single meal. Journal of Experimental Biology 209, 1883–1893, https://doi.org/10.1242/jeb.02205 (2006).
Grosell, M. Intestinal anion exchange in marine fish osmoregulation. J. Exp. Biol. 209, 2813–2827 (2006).
Amirkolaie, A. K., Verreth, J. A. J. & Schrama, J. W. Effect of gelatinization degree and inclusion level of dietary starch on the characteristics of digesta and faeces in Nile tilapia (Oreochromis niloticus (L.)). Aquaculture 260, 194–205 (2006).
Soivio, A., Nikinmaa, M. & Westman, K. The blood oxygen binding properties of hypoxic Salmo gairdneri. Journal of comparative physiology 136, 83–87, https://doi.org/10.1007/bf00688627 (1980).
Tetens, V. & Lykkeboe, G. Blood respiratory properties of rainbow trout,Salmo gairdneri: Responses to hypoxia acclimation and anoxic incubation of blood in vitro. Journal of comparative physiology 145, 117–125, https://doi.org/10.1007/bf00782602 (1981).
Randall, D. J. & Perry, S. F. in Fish Physiology Vol. The Cardiovascular System (eds Hoar, W. S. & Randall, D. J.) 255–300 (Academic Press, 1992).
Lai, J. C. C., Kakuta, I., Mok, H. O. L., Rummer, J. L. & Randall, D. Effects of moderate and substantial hypoxia on erythropoietin levels in rainbow trout kidney and spleen. Journal of Experimental Biology 209, 2734–2738, https://doi.org/10.1242/jeb.02279 (2006).
Bucking, C. & Wood, C. M. The alkaline tide and ammonia excretion after voluntary feeding in freshwater rainbow trout. Journal of Experimental Biology 211, 2533–2541, https://doi.org/10.1242/jeb.015610 (2008).
Claiborne, J. B., Edwards, S. L. & Morrison Shetlar, A. I. Acid-base regulation in fishes: cellular and molecular mechanisms. J. Exp. Zool. 293, 302–319 (2002).
Cameron, J. N. Regulation of blood pH in teleost fish. Respiration physiology 33, 129–144 (1978).
Claireaux, G., Thomas, S., Fievet, B. & Motais, R. Adaptive respiratory responses of trout to acute hypoxia. II. Blood oxygen carrying properties during hypoxia. Respiration physiology 74, 91–98, https://doi.org/10.1016/0034-5687(88)90143-0 (1988).
Marinsky, C. A., Houston, A. H. & Murad, A. Effect of hypoxia on hemoglobin isomorph abundances in rainbow trout. Salmo gairdneri. Canadian Journal of Zoology 68, 884–888, https://doi.org/10.1139/z90-128 (1990).
Fries, C. R. Effects of environmental stressors and immunosuppressants on immunity in Fundulus heteroclitus. Am. Zool. 26, 271–282 (1986).
Sadoul, B., Leguen, I., Colson, V., Friggens, N. C. & Prunet, P. A multivariate analysis using physiology and behavior to characterize robustness in two isogenic lines of rainbow trout exposed to a confinement stress. Physiology & Behavior 140, 139–147, https://doi.org/10.1016/j.physbeh.2014.12.006 (2015).
Ultsch, G. R., Ott, M. E. & Heisler, N. Standard metabolic rate, critical oxygen tension, and aerobic scope for spontaneous activity of trout (Salmo gairdneri) and carp (Cyprinus carpio) in acidified water. Comparative Biochemistry and Physiology Part A: Physiology 67, 329–335, https://doi.org/10.1016/S0300-9629(80)80004-1 (1980).
Folch, J., Lees, M. & Sloane-Stanley, G. H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226, 497–509 (1957).
Abele, D. & Puntarulo, S. Formation of reactive species and induction of antioxidant defence systems in polar and temperate marine invertebrates and fish. Comparative Biochemistry and Physiology - A Molecular and Integrative Physiology 138, 405–415, https://doi.org/10.1016/j.cbpb.2004.05.013 (2004).
Van Bussel, W. et al. Accurate Determination of Titanium as Titanium Dioxide for Limited Sample Size Digestibility Studies of Feed and Food Matrices by Inductively Coupled Plasma Optical Emission Spectromety With Real-Time Simultaneous InternalStandardization. Anglais 31, 81–88 (2010).
Schrama, J. W. et al. Dietary nutrient composition affects digestible energy utilisation for growth: A study on Nile tilapia (Oreochromis niloticus) and a literature comparison across fish species. British Journal of Nutrition 108, 277–289, https://doi.org/10.1017/S0007114511005654 (2012).
Acknowledgements
We thank Menno ter Veld and the staff at the aquatic research facility “De Haar Vissen” of Wageningen University for their technical support in conducting the experiment. We also would like to thank Ronald Booms and Tino Leffering for their assistance during sampling and laboratory work (WIAS, Wageningen University). The aquatic metabolic unit used in this study was cofounded by The Netherlands Organization for Scientific Research (code 805-34.025). Currently it is part of a large-scale EU research facility program. The present study was funded by the EU-FP7 project AQUAEXCEL (262336). L.J. Magnoni is currently supported by a FCT research position with exploratory grant IF/01314/2014/CP1231/CT0001 from Portugal. L.J. Magnoni also wishes to acknowledge WIAS Wageningen University for providing a research fellowship.
Author information
Authors and Affiliations
Contributions
E.E., P.P., I.G. and J.W.S. conceptualized the study. L.J.M., E.E., I.L., P.P., I.G. and J.W.S. were involved in the methodology and formal analysis. L.J.M., E.E., R.O.A.R. and J.W.S. were involved in writing the original draft. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Magnoni, L.J., Eding, E., Leguen, I. et al. Hypoxia, but not an electrolyte-imbalanced diet, reduces feed intake, growth and oxygen consumption in rainbow trout (Oncorhynchus mykiss). Sci Rep 8, 4965 (2018). https://doi.org/10.1038/s41598-018-23352-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-23352-z
This article is cited by
-
Caffeine modulates brain purinergic signaling in Nile tilapia (Oreochromis niloticus) under hypoxia conditions: improvement of immune and inflammatory responses
Fish Physiology and Biochemistry (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.