Abstract
This paper describes a low temperature, enzymatic route to induce fibrillar structures in a protein solution. The route comprises two steps. First, β-lactoglobulin was hydrolyzed into peptides at pH 8 and 37 °C with the enzyme AspN endoproteinase, which resulted in the formation of random aggregates. After hydrolysis, the pH was lowered to 2. As a result, long fibrillar aggregates were formed which was observed using transmission electron microscopy and Thioflavin T fluorescence measurements.
Similar content being viewed by others
Introduction
Long fibrillar protein aggregates can be obtained by heating (80–90 °C) an aqueous solution of β-lactoglobulin for several hours (2–24 h) at pH 2. The fibrils obtained have a length between 1 to 10 μm, and a thickness of a few nanometers.1–4 We showed previously that the fibrils formed under these conditions consist of peptides originating from intact β-lactoglobulin molecules.5 These peptides were formed due to acid hydrolysis of the bonds between aspartic acid residues and any other amino acid residue. The molecules inside these fibrils are held together by intermolecular β-sheets. The presence of these β-sheets can be monitored using the fluorescent dye Thioflavin T (ThT), which binds to the β-sheets.6,7 Therefore, the ThT fluorescent intensity is often used as an indicator for the presence and concentration of fibrils derived from β-lactoglobulin.8
Since protein hydrolysis turned out to be necessary to obtain the peptides of the fibrils, it can be hypothesized that enzymatic hydrolysis may also lead to the formation of peptides that can form fibrils. Previous studies have shown that enzymatic hydrolysis of β-lactoglobulin can lead to the formation of aggregates, and as a result, gelation occured.9,10 However, long fibrillar aggregates were not observed in these studies.
During the current study, we investigated whether enzymatic hydrolysis of β-lactoglobulin can be applied as a method for the formation of peptides that can act as building blocks for fibrillar aggregates. The enzyme AspN endoproteinase was chosen because this enzyme cleaves the peptide bonds N-terminal to aspartic acid residues,11 which would lead to similar peptides as during the heat-induced acid hydrolysis. β-Lactoglobulin was incubated with the enzyme at pH 8 and 37 °C. We studied the formation of β-lactoglobulin aggregates using this method and investigated the necessity of incubation at pH 2 after the hydrolysis step to induce fibril formation.
Materials and Methods
Unless mentioned otherwise, all chemicals were of analytical grade and purchased from Merck (Darmstadt, Germany), Sigma Aldrich (Steinheim, Germany), or Invitrogen (Carlsbad, CA, USA).
Enzymatic Hydrolysis of β-Lactoglobulin
A 2-wt.% β-lactoglobulin (product no. 61329, Sigma Aldrich) solution was dissolved in a buffer (pH 8) containing 50 mM Tris–HCl and 2 mM zinc acetate. The enzyme AspN endoproteinase (P8104S, New England Biolabs, Ipswich, MA, U.S.A.) was dissolved in Millipore water (0.17 mg/ml) and was added to the β-lactoglobulin solution (10–3 mg enzyme/ ml). The solution was incubated at 37 °C and mixed during the incubation. Samples were taken after various hydrolysis times (t hydrolysis = 0.25–24 h). Subsequently, the samples were quenched to pH 2 by adding an HCl solution (18% v/v). At pH 2 and room temperature, the samples were mixed, and the incubation time at pH 2 was 48 h minus the hydrolysis time. No enzyme was added to a part of the β-lactoglobulin solution, which was used as a control sample (t hydrolysis = 0 h).
High-Performance Size-Exclusion Chromatography
HP-SEC experiments were conducted using an ÄKTA purifier system (GE Healthcare, Uppsala, Sweden) operated by Unicorn software. Three samples (t hydrolysis = 2, 11, and 24 h) were freeze–dried, and ~2 mg of the freeze-dried sample was dissolved in 0.5 ml of a 0.15-M Tris–HCl buffer (pH 8) containing 8 M guanidine chloride and 0.1 M 1,4-dithiothreitol (DTT). After mixing for 45–60 min, 0.215 ml of acetonitrile, containing 2% (v/v) tri-fluoroacetic acid (TFA) was added, followed by further mixing for 45–60 min. After mixing, the samples were centrifuged (18,000 × g, 10 min, 20 °C). Samples of 20 μl were applied onto the column (Shodex Protein KW-803, 300 × 8 mm, Showa Denko K. K., Tokyo, Japan). The column was equilibrated and run with 6 M urea, containing 30% (v/v) acetonitrile and 0.1% (v/v) TFA. The flow rate was 0.2 ml/min, and the absorbance was monitored at 214 nm. The column was calibrated using various proteins with molecular masses between 300 and 67,000 Da.
Transmission Electron Microscopy
Transmission electron microscopy (TEM) pictures were made from the sample with a hydrolysis time of 24 h at pH 8 and of the same sample in which the pH was lowered to 2. TEM grids were prepared by negative staining. A droplet of the sample (50× diluted) was put onto a carbon support film on a copper grid. After 15 s, the droplet was removed with a filter paper. Then, a droplet of 2% uranyl acetate was put onto the grid and removed after 15 s using a filter paper. The micrographs were taken with a JEOL electron microscope (JEM2100, Tokyo, Japan) operating at 100 kV.
Thioflavin T Fluorescence
A Thioflavin T (ThT) solution (18 mg/l) was prepared by dissolving ThT in a 10-mM sodium phosphate buffer (pH 7) containing 150 mM NaCl. The solution was filtered (0.2 μm, Minisart®, Sartorius) to remove undissolved ThT. Samples of 48 μl were added to 4 ml ThT solution. The fluorescence of the samples was measured using a luminescence spectrophotometer (LS50B, Perkin Elmer, Waltham, MA, USA). ThT was excited at a wavelength of 460 nm, and the emission of the sample (I ThT) was measured at 486 nm.
Results
β-Lactoglobulin was hydrolyzed by the enzyme AspN endoproteinase, and samples were taken after different hydrolysis times (t hydrolysis = 0.25–24 h). During the enzymatic hydrolysis, the solution became turbid, suggesting the formation of large random aggregates with a typical dimension larger than the wavelength of light. The samples were then quenched to pH 2 and kept at this pH for 48 h minus the hydrolysis time. When the pH was lowered to 2, the samples became less turbid. The control sample to which no enzyme was added did not become turbid at pH 8 or 2.
Figure 1 shows the high-performance size-exclusion chromatography (HP-SEC) elution profiles of three samples (t hydrolysis = 2, 11, and 24 h) and the elution profile of the control sample (t hydrolysis = 0 h). The control sample eluted around 8.8 ml, which is the same elution volume as β-lactoglobulin, and shows that no hydrolysis occurred in the control sample. The hydrolysis products eluted later than β-lactoglobulin, indicating that they have a smaller molecular mass. As expected, the relative amount of β-lactoglobulin decreased as a function of hydrolysis time. After 24 h of hydrolysis, approximately 80% of β-lactoglobulin was converted into smaller molecules.
HP-SEC elution profiles of β-lactoglobulin solutions after different hydrolysis times (t hydrolysis = 0, 2, 11, and 24 h) and incubation at pH 2 (incubation time = 48 h − t hydrolysis). The secondary x-axis indicates the corresponding molecular mass at the elution volume. The increase in absorbance after 12 ml is due to the elution of guanidine chloride
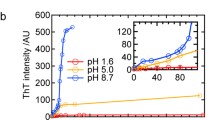
Table 1 shows the fluorescent intensity (I ThT) as a function of hydrolysis time. For each hydrolysis time, the intensity is shown before (immediately after the specific hydrolysis time) and after incubation at pH 2 (t incubation). The fluorescent intensity after hydrolysis increased as a function of hydrolysis time, which indicated that during the enzymatic hydrolysis step at pH 8, aggregates were formed containing a certain amount of intermolecular β-sheets. The fluorescent intensity after incubation at pH 2 was higher for each hydrolysis time than the intensity before incubation at pH 2. This shows that incubation at pH 2 resulted in the formation of more intermolecular β-sheets. The experiments showed no clear correlation between fluorescent intensity and processing time. The complex kinetics of fibril formation, as discussed by Arnaudov et al.,4 in combination with the low fibril concentrations (which leads to difficulties in taking representative samples) probably accounts for the lack of correlation in time.
Figure 2 shows an example of an aggregate that was formed after enzymatic hydrolysis at pH 8. It is a random shaped aggregates with a diameter of ~1 μm. At pH 8, we only observed this type of random aggregates. The presence of these large, random aggregates also explains why the solution became turbid during the enzymatic hydrolysis.
Figure 3a, b shows TEM pictures of the samples after 24 h hydrolysis at pH 8 and 24 h incubation at pH 2. Long fibrillar aggregates that have a length in the order of 1 μm are now visible. Obviously, incubation at acidic pH was necessary to obtain fibrils for these conditions. Apart from the long fibrillar aggregates, random shaped aggregates (~50 nm) were also present in the samples, but the large random aggregates as present at pH 8 seem to have disappeared. To confirm the necessity of protein hydrolysis as a first step for protein fibrillization, TEM pictures were made of the control sample (a 2-wt.% β-lactoglobulin solution that was kept at pH 8 for 24 h without enzyme added, and subsequently incubated at pH 2 for 48 h at room temperature). The control sample did not contain aggregates.
Discussion
This study shows that long fibrillar aggregates from β-lactoglobulin can be obtained by hydrolyzing β-lactoglobulin into peptides by the enzyme AspN endoproteinase. This confirms the results of previous research, which showed that peptides, and not the intact proteins, are the building blocks of fibrillar aggregates of β-lactoglobulin when they were formed by heating at pH 2.5 Fibril formation only took place after incubation at pH 2, and not at pH 8. In our previous study, we explained the presence of specific peptides in the fibrils by their capacity to form β-sheets, the net charge, and the hydrophobicity of those peptides originating from β-lactoglobulin. The fact that lowering the pH to an acidic pH was necessary to obtain fibrils suggests that the positive charge of the peptides is an important factor to obtain fibrils instead of random aggregates. Since a heat treatment above the denaturation temperature was not used during this study, it can also be concluded that this heat treatment is not a prerequisite for transforming peptides into fibrils. Previous work showed already that fibrils of β-lactoglobulin could be formed at room temperature after a short heat treatment of 2 h at 90 °C, during which no fibril formation took place yet.12
At pH 8, the fluorescent intensity increased in time, while random aggregates were formed. At pH 2, random aggregates (smaller in size than at pH 8) and fibrils were observed, which led to a higher fluorescent intensity than at pH 8. In other words, the random aggregates contained β-sheets that resulted in an increase in fluorescent intensity, but the amount of β-sheets was less than the amount obtained at pH 2. Bromley et al.13 also observed that non-fibrillar aggregates of β-lactoglobulin contained β-sheets. ThT fluorescence is thus not a proof for the presence of fibrils, and other methods, such as microscopy (TEM and atomic force microscopy), X-ray diffraction, or flow-induced birefringence,4,8,13 need to be used to confirm the presence of fibrils. Furthermore, the random aggregates might contain a certain amount of peptides that have the capacity to aggregate into fibrils. As a result, the formation of random aggregates leads to a lower availability of peptides to form fibrils.
For the enzymatic hydrolysis, zinc acetate was added to the solution. It has been shown that zinc can have either a positive14 or a negative15 effect on the rate of fibril formation of the non-beta amyloid component (NAC) and β-amyloid of Alzheimer’s disease fibrils. However, the fibril morphology was not influenced by zinc, and the fibril formation itself was not induced by zinc.14
References
D. Durand, J.C. Gimel, T. Nicolai, Aggregation, gelation and phase separation of heat denatured globular proteins. Physica A 304, 253–265 (2002)
W.S. Gosal, A.H. Clark, P.D.A. Pudney et al., Novel amyloid fibrillar networks derived from a globular protein: β-lactoglobulin. Langmuir 18, 7174–7181 (2002)
S.S. Rogers, P. Venema, L.M.C. Sagis et al., Measuring the length distribution of amyloid fibrils: a flow birefringence technique. Macromolecules 38, 2948–2958 (2005)
L.N. Arnaudov, R. de Vries, H. Ippel et al., Multiple steps during the formation of β-lactoglobulin fibrils. Biomacromolecules 4, 1614–1622 (2003)
C. Akkermans, P. Venema, A.J. Van der Goot et al., Peptides are building blocks of heat induced fibrillar protein aggregates of β-lactoglobulin formed at pH 2. Biomacromolecules 9, 1474–1479 (2008)
M.R.H. Krebs, E.H.C. Bromley, A.M. Donald, The binding of Thioflavin-T to amyloid fibrils: localisation and implications. J. Struct. Biol. 149, 30–37 (2005)
H. Naiki, K. Higuchi, M. Hosokawa et al., Fluorometric determination of amyloid fibrils in Vitro using the fluorescent dye, Thioflavine T. Anal. Biochem. 177, 244–249 (1989)
S.G. Bolder, L.M.C. Sagis, P. Venema et al., Thioflavin T and birefringence assays to determine the conversion of proteins into fibrils. Langmuir 23, 4144–4147 (2007)
J. Otte, S.B. Lomholt, R. Ipsen et al., Aggregate formation during hydrolysis of β-lactoglobulin with a Glu and Asp specific protease from Bacillus licheniformis. J. Agric. Food Chem. 45, 4889–4896 (1997)
D. Doucet, E.A. Foegeding, Gel formation of peptides produced by extensive enzymatic hydrolysis of β-lactoglobulin. Biomacromolecules 6, 1140–1148 (2005)
B.G. Grimwood, T.H. Plummer Jr., A.L. Tarentino, Purification and characterization of a neutral zinc endoproteinase secreted by flavobacterium meningosepticum. Arch. Biochem. Biophys. 311, 127–132 (1994)
C. Akkermans, P. Venema, S.S. Rogers et al., Shear pulses nucleate fibril aggregation. Food Biophys. 1, 144–150 (2006)
E.H.C. Bromley, M.R.H. Krebs, A.M. Donald, Aggregation across the length-scales in β-lactoglobulin. Faraday Discuss. 128, 13–27 (2005)
A. Khan, A.E. Ashcroft, V. Higenell et al., Metal accelerate the formation and direct the structure of amyloid fibrils of NAC. J. Inorg. Biochem. 99, 1920–1927 (2005)
C. Ha, J. Ryu, C. Beum Park, Metal ions differentially influence the aggregation and deposition of Alzheimer’s β-amyloid on a solid template. Biochem. 46, 6118–6125 (2007)
Acknowledgement
The authors thank H. Baptist and J. van Lent for their assistance with the TEM analysis. We also thank H. Gruppen and J. Vereijken for the useful discussions about this research. We acknowledge the Dutch research school VLAG, and the Dutch research program MicroNed for the financial support of this research.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Akkermans, C., Venema, P., van der Goot, A.J. et al. Enzyme-Induced Formation of β-Lactoglobulin Fibrils by AspN Endoproteinase. Food Biophysics 3, 390–394 (2008). https://doi.org/10.1007/s11483-008-9094-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11483-008-9094-3