Abstract
Brassica rapa L. is an important vegetable crop in eastern Asia. The objective of this study was to investigate the genetic variation in leaf Zn, Fe and Mn accumulation, Zn toxicity tolerance and Zn efficiency in B. rapa. In total 188 accessions were screened for their Zn-related characteristics in hydroponic culture. In experiment 1, mineral assays on 111 accessions grown under sufficient Zn supply (2 μM ZnSO4) revealed a variation range of 23.2–155.9 μg g−1 dry weight (d. wt.) for Zn, 60.3–350.1 μg g−1 d. wt. for Fe and 20.9–53.3 μg g−1 d. wt. for the Mn concentration in shoot. The investigation of tolerance to excessive Zn (800 μM ZnSO4) on 158 accessions, by using visual toxicity symptom parameters (TSPs), identified different levels of tolerance in B. rapa. In experiment 2, a selected sub-set of accessions from experiment 1 was characterized in more detail for their mineral accumulation and tolerance to excessive Zn supply (100 μM and 300 μM ZnSO4). In this experiment Zn tolerance (ZT) determined by relative root or shoot dry biomass varied about 2-fold. The same six accessions were also examined for Zn efficiency, determined as relative growth under 0 μM ZnSO4 compared to 2 μM ZnSO4. Zn efficiency varied 1.8-fold based on shoot dry biomass and 2.6-fold variation based on root dry biomass. Zn accumulation was strongly correlated with Mn and Fe accumulation both under sufficient and deficient Zn supply. In conclusion, there is substantial variation for Zn accumulation, Zn toxicity tolerance and Zn efficiency in Brassica rapa L., which would allow selective breeding for these traits.
Similar content being viewed by others
Introduction
Zinc (Zn) is an essential micronutrient required by all organisms for its role in many physiological processes as a structural or catalytic component of proteins. Unfortunately Zn deficiency is a widespread problem by affecting humans in case of Zn shortage in food. About 20% of rural children are at risk of inadequate Zn intake in China (Ma et al. in press). Zn deficiency is also affecting crops in case of poor Zn availability in soil. In China Zn deficiency is prevalent on calcareous soil in North China and calcareous alluvial soils of the Middle and Lower Yangtse River valley (Liu 1994). Breeding and growing of crops with high Zn content and Zn efficiency are promising and sustainable approaches to solve the Zn deficiency problems in humans and soil (Cakmak et al. 1996).
Knowledge on genetic variation of Zn accumulation and Zn efficiency is the prerequisite for breeding of Zn content/efficiency-improved crop cultivars. Previous studies on genetic variation of micronutrients were mainly limited to staple food crops, including wheat (Graham et al. 1997), rice (Graham et al. 1999), bean (Beebe et al. 2000) and maize (Banziger and Long 2000). Little is known about micronutrient content in leaves, which is the main edible organ of leafy vegetables (Kopsell et al. 2004). The ability of a genotype to grow and yield well in soils that are too deficient in Zn for a standard cultivar to grow and yield well, is defined as Zn efficiency (Graham et al. 1992). Progress has been made in screening Zn efficient genotypes and understanding the physiological and biochemical mechanisms of Zn efficiency (Reviewed in Hacisalihoglu and Kochian 2003). However, knowledge on Zn efficiency in vegetables is limited (Hacisalihoglu et al. 2004).
Brassica rapa L. comprises several cultivar types producing edible roots, stems, leaves, buds or flowers as vegetables (Gomez-Campo and Prakash 1999). Some of these are the most important vegetables in eastern Asia, especially in China, Korea and Japan, both in terms of production and per capita consumption (Opena et al. 1988). As vegetables are one of the main micronutrient sources of the population in China (Ma et al. in press), we studied B. rapa vegetables to collect more information on the extent of genotypic variation for Zn accumulation and Zn efficiency and their potential for genetic improvement of these traits.
While a shortage of Zn is a problem for plant growth, an excess of Zn is even more detrimental. Zn heavy metal pollution is prevalent in China’s industrialized areas (Liu et al. 2005; Nan and Zhao 2000). B. rapa is not known to be a metal hyperaccumulator and showed a significant decrease in biomass with increased root and shoot Zn concentration upon exposure to toxic Zn levels (Ebbs and Kochian 1997; Coolong and Randle 2003; He et al. 2004). However, in general only one accession was tested in each case. We therefore intended to determine the natural variation for excess Zn tolerance (ZT) among B. rapa germplasm.
The objective of this study is to characterize the genotypic variation for Zn accumulation and Zn response in B. rapa upon exposure to different Zn concentrations. Understanding the range of genotypic variation in Zn accumulation and response to Zn nutritional stress will provide a genetic basis for micronutrient and Zn stress tolerance breeding of B. rapa vegetables and for further genetic studies on Zn accumulation and tolerance to Zn nutritional stress.
Materials and methods
Plant material
To determine the genetic variation of Zn accumulation and response to Zn stress, a total of 188 Brassica rapa accessions belonging to nine cultivar groups (Table 1) were screened. About 184 accessions were obtained from the Institute of Vegetables and Flowers of the Chinese Academy of Agricultural Sciences (IVF-CAAS); two were obtained from the Dutch Crop Genetic Resources Centre (CGN) in Wageningen, and the other two were obtained from Dr. T. Osborn (University of Wisconsin, Madison, USA). About 111 accessions were used for shoot (aboveground tissue) mineral analysis and 158 lines were screened for their tolerance to Zn excess stress. On the basis of their performance in this large scale screening experiment (experiment 1), 15 accessions were selected for a detailed accumulation and tolerance testing (experiment 2) as described below. In experiment 2, six additional accessions were added, which are the parents of additional doubled haploid (DH) populations that are under development.
Plant culture
For experiment 1, three plants for each accession were grown in a greenhouse without climate control in Beijing, China, from mid March till May. The environmental conditions were 20–30°C/10–15°C (day/night temperature), 30,000–40,000 Lux light intensity and 50–60% relative humidity. Seeds were germinated in vermiculite and watered every 3 days with half-strength Hoagland’s nutrient solution after germination. After 14 days (mineral accumulation experiment) or 7 days (Zn tolerance experiment) seedlings were transferred to hydroponic culture trays each containing three individuals from 24 accessions in 20 l half-strength Hoagland’s nutrient solution. The solution was buffered with 2 mM MES (2-morpholinoethanesulphonic acid) at pH 5.5. A concentration of 2 μM ZnSO4 was used as sufficient Zn supply. Nutrient solutions were replaced once a week until harvesting. After 7 days at sufficient Zn, plants for the ZT experiment were transferred to excess Zn nutrient solution containing 800 μM ZnSO4, and were exposed for 14 days. The solution was refreshed after 1 week.
For experiment 2, plants were grown in a climate-controlled growth cabinet set at 75% humidity and 22/16°C (16 h/8 h) day/night temperature regime. Seeds were germinated in fertilized potting soil watered with tap water. Seedlings were transferred to hydroponic solution after 14 days for the mineral accumulation experiment or after 7 days for the ZT and Zn efficiency experiments. For the mineral accumulation experiment plants were grown for 14 days in medium with sufficient Zn (2 μM ZnSO4). For the ZT experiment plants were first grown for 7 days in medium with sufficient Zn (2 μM ZnSO4) before exposure to excess Zn for 14 days. Instead of the very high concentration of 800 μM ZnSO4, plants were transferred to 100 and 300 μM ZnSO4 as excess Zn concentrations and to 2 μM ZnSO4 as the sufficient Zn control. For the Zn efficiency experiment, one-week-old germinated seedlings were transferred directly to a nutrient solution without ZnSO4 or with 2 μM ZnSO4 as control and grown for 15 days before assessment. For each line three pots were used with one plant per pot for the mineral accumulation experiment and three plants per pot for the Zn efficiency and ZT experiments. In all these experiments the nutrient solutions were refreshed twice a week.
Mineral determination
In experiment 1, shoots were harvested from plants with a similar size after 27–36 days of growth. For each accession shoots from 2 to 3 individual plants were combined in one sample. Harvested shoots were washed with de-ionized water and lyophilized. Samples were ground by mortar and pestle before wet-digestion in concentrated HNO3:HClO4 (87:13, V/V) subsequently at 60°C for 3 h, 100°C for 1 h, 120°C for 1 h and 195°C for 2.5 h. The digests were diluted with 5 ml 20% HCl and deionized H2O to a final volume of 20 ml before analysis by inductively coupled plasma–atomic emission spectrometer (ICP-AES) (Leeman-DRE DR6009, USA) at the IVF-CAAS in Beijing.
In experiment 2, shoots and roots were harvested separately per plant. After oven-drying at 65°C for 3 days, shoot and root dry biomass were measured. Shoot samples were ground by mortar and pestle before wet-digestion in concentrated HCl:HNO3 (1:4, V/V) at 140°C for 7 h. Mineral assays were performed by using a flame Atomic Absorption Spectrometer (AAS) (model 1100, Perkin–Elmer) at the Vrije Universiteit, Amsterdam. Seed mineral content of the 21 accessions used for experiment 2 was determined in samples of about 100 mg ground seeds. Seed mineral determination assays were as described for shoots.
Zn efficiency and tolerance
Shoots and roots were harvested separately and dried at 65°C for 3 days to determine their dry biomass. Zn efficiency (ZE) was calculated for shoots and roots based on relative biomass production using the following calculation: ZE (%) = [dry biomass at 0 μM Zn/dry biomass at 2 μM Zn] * 100%.
In the first ZT experiment, a ranked set of five Toxicity Symptom Parameters (TSPs) representing different levels of deterioration of the leaves was used to score plant response after exposure to 800 μM ZnSO4: 1 = slight chlorosis of leaves, plant is still growing; 2 = chlorosis of leaves; 3 = severe chlorosis of leaves, leaves started withering; 4 = most of the leaves seared; 5 = plant has died. Accessions with mean TSP values below 2 were classified as Zn tolerant and above 4 as Zn sensitive.
For the second ZT experiment, ZT was calculated in two different ways. One calculation was made based on dry root or shoot biomass: ZTbiomass (%) = [dry biomass at toxic level Zn/dry biomass at 2 μM Zn] * 100%. Another calculation was based on root elongation during exposure. For this analysis, roots were dyed with active charcoal before transferring plants to excess Zn medium. Root elongation during exposure (non-stained part of the root) was measured according to Schat and Ten Bookum (1992): ZTroot (%) = [root elongation at toxic level Zn/root elongation at 2 μM Zn] * 100%.
Statistics
Statistical analyses of metal concentration and root length were conducted using one-way ANOVA followed by the Student-Neuman-Keuls posthoc analysis (SigmaStat, SPSS Science, Chicago, IL, USA). The variation within the mean is presented as the standard error. Significance of correlation was determined using simple linear regression. We considered differences significant at P ≤ 0.05.
Results
Zn, Fe and Mn accumulation
When examining the shoot Zn concentration for 111 accessions, belonging to seven cultivar groups of B. rapa, grown in hydroponic culture with sufficient Zn supply for about 4 weeks, large variations were found between accessions, ranging from 23.2 to 155.9 μg Zn g−1 d. wt. (Fig. 1a). Accessions with a Zn concentration lower than 50 μg g−1 d. wt., between 50 and 100 μg g−1 d. wt. and above 100 μg g−1 d. wt. accounted for respectively 37%, 56% and 7% of the total. The same samples were used to determine the Fe and Mn concentrations. The Fe concentration varied from 60.3 to 350.1 μg g−1 d. wt. (Fig. 1b). Accessions with a Fe concentration lower than 100 μg g−1 d. wt., between 100 and 200 μg g−1 d. wt. and above 200 μg g−1 d. wt. accounted respectively for 28%, 71% and 1% of the total. The Mn concentration ranged from 20.9 to 53.3 μg g−1 d. wt. (Fig 1c). The proportions of accessions with a Mn concentration lower than 30 μg g−1 d. wt., between 30 and 50 μg g−1 d. wt. and above 50 μg g−1 d. wt. were respectively 28%, 69% and 3% of the total. There was no significant difference in average Zn or Fe concentration between the different cultivar groups, however the average Mn concentrations in Wutacai and Mizuna accessions were significantly higher compared to those of the other cultivar groups (Table 2). Zn concentration varied most in Wutacai with a variation coefficient as high as 60%.
Genotypic variation of shoot Zn (a), Fe (b) and Mn (c) concentrations of 111 B. rapa accessions grown in half-strength Hoagland’s nutrient solution containing 2 μM Zn for 27–36 days. Data are based on a mixed sample of 2–3 plants per accession. Accessions are ordered according to increasing mineral content
Five accessions with low Zn concentration (on average 40 μg g−1 d. wt.), four accessions with moderate Zn concentration (on average 67 μg g−1 d. wt.) and six accessions with high Zn concentration (on average 135 μg g−1 d. wt.) were selected for further confirmation in a subsequent experiment, with plants grown under climate-controlled conditions (Experiment 2). Six additional accessions, which are the parents of recently developed DH populations, were also included. In experiment 2, the range of Zn concentration was slightly less (43.5–135.0 μg g−1 d. wt.) than in experiment 1 (Table 3). The mean Zn concentration was comparable in both experiments. In general, the Fe concentration was lower in experiment 2 compared to experiment 1 (ranging from 40.4 to 70.6 μg g−1 d. wt.), whereas it was the reverse for the Mn concentration (ranging from 44.5 to 113.4 μg g−1 d. wt.). The data from the two experiments were not significantly correlated (R 2 = 0.10 for Zn, 0.06 for Fe and 0.24 for Mn). When comparing the 21 accessions, the Fe concentrations were not significantly different, but there were significant differences for the Zn and Mn concentrations (Table 3). The Zn, Fe and Mn concentrations of these accessions were positively correlated (Fig. 2). The correlation between Zn and Mn (R 2 = 0.58, P ≤ 0.001) was much higher than that of Zn and Fe (R 2 = 0.19, P ≤ 0.05), however, when excluding the data of outlier accession L144 from the data set, the correlation between Zn and Fe concentrations was significant at P ≤ 0.005 (R 2 = 0.47). Omitting this accession from the correlation analysis did not affect the significance level for the correlation between Zn and Mn concentrations (R 2 = 0.67, P ≤ 0.001) or Fe and Mn concentrations (R 2 = 0.43, P ≤ 0.005). In addition to shoot mineral concentration, the seed weight, seed mineral content and plant biomass were determined for these accessions (Table 4). When comparing the shoot mineral concentration to the dry shoot or root biomass (Table 4), shoot Fe concentration was positively correlated with dry shoot biomass (R 2 = 0.32, P ≤ 0.05) and root biomass (R 2 = 0.34, P ≤ 0.05), while neither Zn concentration nor Mn concentration was correlated with biomass. There was no significant correlation between shoot concentration and content per seed for Zn and Fe (Table 4), however, a significant correlation was found for seed Mn content and shoot Mn concentration (R 2 = 0.25, P ≤ 0.05).
Correlations between shoot Zn, Fe and Mn concentrations of 21 selected accessions grown in half-strength Hoagland’s nutrient solution with 2 μM ZnSO4 for 14 days. (a) Correlation between shoot Zn and Fe concentrations. (b) Correlation between shoot Zn and Mn concentrations. (c) Correlation between shoot Fe and Mn concentrations. Correlation between shoot Zn and Fe concentrations was significant at P ≤ 0.005 (R 2 = 0.47) when excluding the low Fe outlier L144. *, ** and *** are statistically significant at P ≤ 0.05, P ≤ 0.005 and P ≤ 0.001 levels respectively. Significance was determined by simple linear regression; R 2 = squared linear regression coefficient
Zn tolerance
Although B. rapa is not known to be particularly tolerant to excess Zn exposure, we assessed the initial set of 158 accessions for their tolerance to 800 μM ZnSO4 exposure for 14 days (Experiment 1). Tolerance was determined using TSPs, and tolerant, average and sensitive accessions accounted for respectively 8%, 45% and 46% of the total (Fig. 3). Exposure to 800 μM ZnSO4 is rarely encountered by plants in the field. To determine if comparable results could be obtained when exposing plants to less extreme Zn concentrations, two tolerant accessions (L56w and L86w), three average accessions (L58w, L64w and L203w) and one sensitive accession (L66w) were used in experiment 2. In this experiment, plants were exposed to 2 μM ZnSO4 as normal Zn supply and 100 and 300 μM ZnSO4 as excess Zn supply. Zn tolerance was determined in terms of dry biomass production rather than with TSPs (Table 5). As expected, biomass was reduced when plants were grown at high Zn concentrations and the growth inhibition increased along with the increase in Zn concentration (Table 5). ZT based on shoot dry biomass varied almost 2-fold among accessions at both 100 μM and 300 μM ZnSO4. A comparable range was found for ZT based on root dry biomass. In line with the initial selection based on TSP values, the two tolerant accessions L56w and L86w maintained a high relative shoot growth at 300 μM Zn (94%), whereas the sensitive accession L66w showed only 50% relative shoot growth at 300 μM Zn (Table 5). The range of relative growth was different between roots and shoots (Table 5). The 300 μM Zn treatment induced a drastic decrease in root biomass by 65–82%, while this induced only a moderate reduction in shoot biomass of between 6 and 50%.
Excess Zn stress response of 158 B. rapa accessions grown in half-strength Hoagland’s nutrient solution with 800 μM Zn for 14 days. TSP (Toxic Symptom Parameter): 1 = slight chlorosis, still growing; 2 = chlorosis; 3 = severe chlorosis, leaves started withering; 4 = most of the leaves seared; 5 = dead. Values are means of two or three plants per accession
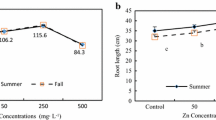
When determining ZT in terms of root elongation, it became clear that the effect on root biomass was not reflected by an effect on root length (Table 6), as the relative growth calculated for the increase of maximum root length is not consistent with the previously determined ZT based on root dry biomass. Root elongation of accessions L56w and L86w, that showed the highest relative root dry biomass with respectively 35% and 26% of the control, was strongly inhibited at 300 μM Zn to respectively 11% and 9% of the control. Root elongation of all six accessions was inhibited at 100 μM Zn and this inhibition was enhanced at 300 μM Zn. The difference in root elongation between accessions decreased with increased Zn concentration. When plants were grown at 300 μM Zn there was no significant difference in the mean root elongation among the six accessions.
Zn efficiency
In experiment 2, the six accessions (L56w, L58w, L64w, L66w, L86w, L203w) used in the ZT experiment were also examined for their Zn efficiency (ZE), i.e. the ability to grow under low Zn supply. Plants began to show typical symptoms of Zn deficiency, such as interveinal chlorosis, a purple stem and reduced growth, already after growing for 1 week in Zn deficient medium. After 2 weeks, the difference in phenotype compared to untreated plants was easily distinguished by eye with the exception of accessions L64w and particularly L56w, which appeared comparatively healthy (Fig. 4).
Both root and shoot dry matter production was reduced in all accessions due to Zn deficiency (Table 7), but this reduction was much less in L64w for both shoot (69.8%) and root (98.0%). L64w is therefore considered to be the most zinc efficient accession of the six accessions tested. ZE varied more for shoot biomass (2.6-fold) than for root biomass (1.8-fold). In addition to ZE we also examined the relative root:shoot biomass ratio (RSR) for the six accessions. Zn deficiency enhanced the RSR in most accessions and thus has a stronger effect on shoot biomass production than on root biomass production. However, since the most Zn efficient accession L64w has a very similar RSR as the least Zn efficient accession L86w, there does not seem to be a very strong correlation between ZE and RSR. When comparing ZE based on shoot biomass, root biomass or RSR with seed weight and mineral content per seed (Table 4), no significant correlation was found (data not shown).
Shoot Zn concentration did not differ among the six accessions after plants were grown under Zn deficient condition for 15 days with a reduction of 76–84% (Table 8). There was no significant correlation between ZE and the shoot Zn concentration at 0 μM or 2 μM Zn (data not shown). In general, both shoot Fe and Mn concentrations were increased under Zn deficient condition when compared to Zn sufficient condition (Table 8). Four out of six accessions showed a significant increase in shoot Mn concentration, while two of these accessions also increased significantly in Fe concentration. There was no correlation between ZE and shoot Mn or Fe concentration of plants grown at 0 or 2 μM Zn (data not shown).
Discussion
In total 117 B. rapa accessions were screened for Zn, Mn and Fe accumulation characteristics as a general survey for genotypic variation among B. rapa vegetables. This survey showed that there is considerable genotypic variation for shoot Zn, Mn and Fe concentration in B. rapa. This variation is not limited to one or a few cultivar groups and there is also no clear correlation between mineral concentration and cultivar group. When the selected accessions were re-examined at a second location, different results were obtained for some of these accessions and in general the correlation between locations was lower than expected. This does not reflect errors in sampling or measuring mineral concentrations, but largely illustrates the difficulty associated with studying a trait that is easily affected by genotype × environment interactions, which is often the case for mineral accumulation. Genetic variation within accessions is another factor that may have caused differences in mineral concentration. Although the accessions had been propagated for some generations in the resource collection they originated from, the occurrence of self-incompatibility, which is common in B. rapa, is expected to maintain some genetic variation within each accession.
Previous studies revealed variation ranges of 10–60 μg g−1 for Zn and 10–90 μg g−1 for Fe in seeds (Beebe et al. 2000; Graham et al. 1999; Graham et al. 1997; Banziger and Long 2000). Kopsell et al. (2004) reported that leaf Zn concentration based on fresh weight ranged from 29.1 to 71.9 mg g−1and Fe concentration ranged from 53.1 to 114.2 mg g−1 in B. oleracea vegetables. In the present study a wider variation range (7-fold) and higher highest concentration were found for Zn and Fe in B. rapa shoots. Of course this may reflect a physiological difference in the accumulation process between shoot and seed. Shoot accumulation largely depends on xylem transport, whereas seed accumulation requires additional phloem transport. Also different from screening plants in hydroponic culture, as was done in our study, the investigations on above staple crops were all carried out in soil, which might cause lower mineral availability. Seed weight and mineral content influence plant growth at the early vegetative stage (Rengel and Graham 1995a) and therefore affect shoot mineral accumulation. In the present study the correlation of Mn concentration in shoot and Mn content in seed supports the previous conclusion, however, there was no correlation between shoot concentration and seed content for Zn and Fe. Thus, the variation for Zn and Fe concentration as observed in this study suggests that there is sufficient genetic variation to dissect the genetic mechanism controlling shoot Zn and Fe accumulation by quantitative trait locus (QTL) analysis and/or to improve Zn and Fe content in B. rapa vegetables by breeding.
In addition to growth under sufficient Zn supply we also studied the response to Zn excess and deficiency in B. rapa. Both relative shoot and root growth have been suggested as good indices of tolerance to excess Zn in different species (Bert et al. 2000; Escarre et al. 2000; Meerts and Van Isacker 1997; Schat and Ten Bookum 1992; Yang et al. 2004). The comparable range in variation of ZT we observed, which was based on dry shoot and root biomass, suggests that the same holds for B. rapa. However, ZT determined by maximum root length did not correlate with the ZT determined by dry biomass, although both root elongation and biomass increase were inhibited in a concentration-dependent manner when exposed to toxic Zn concentrations. Our results thus support the suggestion by Ebbs and Kochian (1997) that the toxic effect of excess Zn on the root development in Brassica ssp. has more effect on lateral root elongation than on lateral root density.
To examine the response to deficient Zn exposure, relative shoot growth was reported as a suitable index to determine Zn efficiency (Cakmak et al. 1999; Grewal et al. 1997; Hacisalihoglu et al. 2004; Rengel and Römheld, 2000), although also the relative root:shoot biomass ratio (RSR) has been suggested to be adequate (Rengel and Graham, 1995b). In the present study, both the relative shoot and relative root biomass index were effective in distinguishing differences in Zn efficiency. Accession L64w clearly stood out as the least affected by low Zn supply when compared to the other accessions. The RSR generally increases under Zn deficiency as an initial response to Zn deficiency (Grewal et al. 1997; Khan et al. 1998; Loneragan et al. 1987). Higher RSRs correlating with Zn efficiency have also been reported for B. napus and B. juncea (Grewal et al. 1997). A comparable result was obtained in the present study, with the exception that, based on biomass production, the relatively Zn inefficient accession L86w had a similarly high RSR as the Zn efficient accession L64w. Considering this, the RSR does not seem to be the optimal Zn efficiency index for B. rapa.
While variation for both ZT and Zn efficiency was observed among the six accessions, there was no significant correlation between these traits. The absence of correlation between Zn efficiency and seed weight, seed mineral content or shoot mineral concentration also indicates that Zn efficiency is genetically independent from these traits. When grown under Zn deficiency, the limited Zn supply resulted in an almost uniform shoot Zn concentration for all accessions that was below 10 μg −1 d. wt. Since a leaf Zn concentration below 10–15 μg g−1 d. wt. is considered to be the critical Zn deficiency level for normal plant growth (Marschner 1995), this explains the negative effect of the Zn deficiency treatment on growth of the B. rapa accessions.
In addition to an effect on Zn concentration, we found that when plants were grown under Zn deficient conditions, both Fe and especially Mn concentration in shoots increased, comparable to what has been found in wheat (Rengel and Graham 1996) or Arabidopsis (van de Mortel et al. 2006). Most of the known metal transporters belong to large gene families covering a broad range of metal specificities. Several of the Zn transporters can also transport Fe or Mn (Connolly et al. 2002; Mills et al. 2003; Vert et al. 2001). Decreased shoot Fe and Mn concentration was found for Brassica plants grown in high-level Zn conditions (Ebbs and Kochian 1997). This is also in line with the correlations we observed between shoot Zn, Fe and Mn concentrations when plants were grown under sufficient Zn supply (Fig. 2). In both experiments (sufficient and deficient Zn supply), the strongest correlation was found between Zn and Mn, suggesting that Zn and Mn accumulation share more common elements than Fe and Mn or Fe and Zn accumulation.
Another observation was that the difference in Zn efficiency based on biomass was not fully reflected in the visual appearance of the accessions. Accessions L64w and L56w seemed to suffer little from deficient Zn supply in terms of plant size and degree of leaf senescence or yellowing of the leaves (Fig. 4). However, when scored for biomass production, L56w did not perform better than the other accessions with a comparable ZE value. A similar difference in visible appearance and biomass production was previously found for wheat (Genc and McDonald 2004). This suggests that the plant response to low zinc can act at different levels and the effect on biomass production is not always easily visible by eye. It also shows that visual selection of Zn efficient plant genotypes by breeders may be misleading with respect to yield.
Based on the screening of a large set of B. rapa accessions, we conclude there is substantial genotypic variation for Zn, Fe and Mn accumulation and for tolerance to excessive or deficiency inducing levels of Zn. Relative shoot and root growth calculated on dry biomass yield are suitable indices both for the evaluation of excess ZT and for Zn efficiency. There is a close relationship between Zn, Mn and Fe accumulation. Our results underline that breeding for improved Zn content, whether or not in combination with enhanced fertilization with Zn, is likely to substantially increase the Zn content of B. rapa vegetables and thus offer a desirable Zn supplementation to a vegetarian human diet.
References
Banziger M, Long J (2000) The potential for increasing the iron and zinc density of maize through plant breeding. Food Nutr Bull 21:397–400
Beebe S, Gonzalaz AV, Rengifo J (2000) Research on trace minerals in the common bean. Food Nutr Bull 21:387–391
Bert V, Macnair MR, DeLaguerie P, Saumitou-Laprade P, Petit D (2000) Zinc tolerance and accumulation in metallicolous and non metallicolous populations of Arabidopsis halleri (Brassicaceae). New Phytol 146:225–233
Cakmak I, Sari N, Marschner H, Kalayci M, Yilmaz A, Eker S, Gülüt KY (1996) Dry matter production and distribution of Zn in bread and durum wheat genotypes differing in Zn efficiency. Plant Soil 180:173–181
Cakmak I, Tolay I, Ozdemir A, Ozkan H, Oztürk L, Kling CI (1999) Differences in zinc effciency among and within diploid, tetraploid and hexaploid wheats. Ann Bot 84:163–171
Connolly EL, Fett JP, Guerinot ML (2002) Expression of the IRT1 metal transporter is controlled by metals at the levels of transcript and protein accumulation. Plant Cell 14:1347–1357
Coolong TW, Randle WM (2003) Zn concentration in hydroponic solution culture influences zinc and sulfur accumulation in Brassica rapa L. J Plant Nutr 26:949–959
Ebbs SD, Kochian LV (1997) Toxicity of zinc and copper to Brassica species: Implications for phytoremediation. J Environ Qual 26:776–781
Escarre J, Lefebvre C, Gruber W, Leblanc M, Lepart J, Riviere Y, Delay B (2000) Zinc and cadmium hyperaccumulation by Thlaspi caerulescens from metalliferous and nonmetalliferous sites in the Mediterranean area: implications for phytoremediation. New Phytol 145:429–437
Genc Y, McDonald GK (2004) The potential of synthetic hexaploid wheats to improve zinc efficiency in modern bread wheat. Plant Soil 262:23–32
Gomez-Campo C, Prakash S (1999) Origin and domestication. In: Gomez-Campo C. (ed) Biology of Brassica Coenospecies. Elsevier Science BV, Amsterdam, The Netherlands, pp 33–52
Graham RD, Ascher JS, Hynes SC (1992) Selecting Zn efficient cereal genotypes for soils of low Zn status. Plant Soil 146:241–250
Graham RD, Senadhira D, Ortiz-Monasterio I (1997) A strategy for breeding staple-food crops with high micronutrient density. Soil Sci Plant Nutr 43:1153–1157
Graham RD, Senadhira D, Beebe S, Iglesias C, Monasterio I (1999) Breeding for micronutrient density in edible portions of staple food crops: conventional approaches. Field Crop Res 60:57–80
Grewal HS, Stangoulis JCR, Potter TD, Graham RD (1997) Zinc efficiency of oilseed rape (Brassica napus and B. juncea) genotypes. Plant and Soil 191:123–132
Hacisalihoglu G, Kochian LV (2003) How do some plants tolerate low levels of soil zinc? Mechanisms of zinc efficiency in crop plants. New Phytol 159:341–350
Hacisalihoglu G, Ozturk L, Cakmak I, Ross MW, Kochian LV (2004) Genotypic variation in common bean in response to zinc deficiency in calcareous soil. Plant Soil 259:71–83
He PP, Lv XC, Wang GY (2004) Effect of Se and Zn supplementation on the antagonism against Pb and Cd in vegetables. Environ Int 30:167–172
Khan HR, McDonald GK, Rengel Z (1998) Chickpea genotypes differ in their sensitivity to Zn deficiency. Plant Soil 198:11–18
Kopsell DE, Kopsell DA, Lefsrud MG, Curran-Celentano J (2004) Variability in elemental accumulations among leafy Brassica oleracea cultivars and selections. J Plant Nutri 27:1813–1826
Liu Z (1994) Regularities of content and distribution of zinc in soils of China. Scientia Agricultura Sinica 27:30–37
Liu WH, Zhao JZ, Ouyang ZY, Soderlund L, Liu GH (2005). Impacts of sewage irrigation on heavy metal distribution and contamination in Beijing, China. Environ Int 31:805–812
Loneragan JF, Kirk GJ, Webb MJ (1987) Translocation and function of Zn in roots. J Plant Nutr 10:1247–1254
Marschner H (1995). Mineral nutrition of higher plants. Academic Press. London, UK, p 352
Ma G, Li Y, Jin Y, Du S, Kok FJ, Yang X. Assessment of zinc intake adequency and food source of people in China. Public Health Nutrition (in press)
Meerts P, Van Isacker N (1997) Heavy metal tolerance and accumulation in metallicolous and non-metallicolous populations of Thlaspi caerulescens from continental. Europe. Plant Ecol 133:221–231
Mills RF, Krijger GC, Baccarini PJ, Hall JL, Williams LE (2003) Functional expression of AtHMA4, a P1B-type ATPase in the Zn/Co/Cd/Pb subclass. Plant J 35:164–175
Nan ZR, Zhao CY (2000) Heavy metal concentrations in grey calcareous soils of Baiyin region, Gansu province, P.R.China. Water Air Soil Pollut 118:131–141
Opena RT, Kuo CG, Yoon JY (1988) Breeding and seed production of Chinese cabbage in the tropics and subtropics. Technical Bulletin No. 17. AVRDC, Shanhus, Taiwan
Rengel Z, Graham RD (1995a) Importance of seed Zn content for wheat growth on Zn-deficient soil. I Vegetative growth. Plant Soil 173:259–266
Rengel Z, Graham RD (1995b) Wheat genotypes differ in Zn efficiency when grown in chelate-buffered nutrient solution. I. Growth. Plant Soil 176:307–316
Rengel Z, Graham RD (1996) Uptake of zinc from chelate-buffered nutrient solutions by wheat genotypes differing in zinc efficiency. J Exp Bot 47:217–226
Rengel Z, Römheld V (2000) Differential tolerance to Fe and Zn deficiencies in wheat germplasm. Euphytica 113:219–225
Schat H, Ten Bookum WM (1992) Genetic control of copper tolerance in Silene vulgaris. Heredity 63:219–229
van de Mortel JE, Almar Villanueva L, Schat H, Kwekkeboom J, Coughlan S, Moerland PD, Ver Loren van Themaat E, Koornneef M, Aarts MGM (2006) Large expression differences in genes for iron and zinc homeostasis, stress response, and lignin biosynthesis distinguish roots of Arabidopsis thaliana and the related metal hyperaccumulator Thlaspi caerulescens. Plant Physiol 142:1127–1147
Vert G, Briatt JF, Curie C (2001) Arabidopsis IRT2 gene encodes a root periphery iron transporter. Plant J 26:181–189
Yang XE, Long XX, Ye HB, He ZL, Calvert DV, Stoffella PJ (2004) Cadmium tolerance and hyperaccumulation in a new Zn-hyperaccumulating plant species (Sedum alfredii Hance). Plant Soil 259:181–189
Acknowledgements
We thank Dr. Xixiang Li (Institute of Vegetables and Flowers of the Chinese Academy of Agricultural Sciences, Beijing, China); the Dutch Crop Genetic Resources Centre (CGN) (Wageningen, NL) and Dr. T. Osborn (University of Wisconsin, Madison, USA) for kindly supplying the accessions used in this study. This research is supported by the Wageningen University-Chinese Academy of Agricultural Sciences INREF Joint PhD Training Programme, the Centre for Biosystems Genomics and The Opening Lab of Vegetable Genetics and Physiology of the Ministry of Agriculture, P. R. China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Wu, J., Schat, H., Sun, R. et al. Characterization of natural variation for zinc, iron and manganese accumulation and zinc exposure response in Brassica rapa L.. Plant Soil 291, 167–180 (2007). https://doi.org/10.1007/s11104-006-9184-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-006-9184-2