Abstract
The utilization of maize stover as a substrate for bioenergy production demands the development of dual-purpose hybrid varieties combining both, optimal grain yield and improved biomass processing amenability. In this study, our objectives were to assess how contrasting environments influence the expression of cell wall composition and bioconversion traits relevant to cellulosic fuel production, and to study how these traits are inherited in hybrid combinations. To this end, a panel of maize double haploid (DH) lines and their corresponding test-cross (TC) offspring were tested under different locations (primarily in the Netherlands) and characterized for a variety of cell wall compositional and bioconversion features relevant to cellulosic fuel production. Overall, the DH and TC sets displayed extensive genotypic diversity in cell wall composition, polymeric ultrastructure and bioconversion characteristics. Heritability for the different traits was generally high (h 2 > ~0.60); essentially implying that systematic differences between genotypes remained constant across divergent environmental conditions. Moreover, correlations between the performance of DH lines and related TC hybrids were significant and favorable for most investigated traits. Strong associations (r > ~0.50) were especially prominent for cell wall lignin content, degree of substitution of cell wall glucuronoarabinoxylans and cell wall convertibility following pretreatment and enzymatic hydrolysis. In conclusion, complex cell wall bioconversion traits constitute accessible and reliable selection criteria for incorporation in modern breeding programs seeking to advance bio-based maize hybrid varieties. The high heritability and environmental stability of these traits guarantee high selection efficacy during the development of superior DH/inbred material; and their predominantly additive nature prescribe that preliminary selection at the inbred level will guarantee similar correlated genetic gains in hybrid breeding.
Similar content being viewed by others
Introduction
As the most important crop worldwide in relation to global acreage, maize is envisioned to play an essential role in the wide-scale realization and commercialization of cellulosic fuel technologies (van der Weijde et al. 2013). In fact, with an unrivalled production and distribution infrastructure, and nearly 1300 million tons of dry stover available annually, maize is warranted to become the first large-scale lignocellulosic crop in the industry (Torres et al. 2014). Conceivably, the intensive utilization of maize stover as a substrate for bioenergy production will create a demand for dual-purpose hybrid varieties combining both, optimal grain yield and improved stover quality (Torres et al. 2014; van der Weijde et al. 2013).
In this context, a pivotal objective for breeding “bioenergy” maize is improving complex cell wall characteristics influencing the industrial quality of its biomass (Torres et al. 2014). Numerous studies have demonstrated that bioenergy crops diverging in cell wall constitution exert a differential influence on the technical efficacy of biomass-to-fuel conversion platforms (Dien et al. 2009; Fu et al. 2011; Torres et al. 2013, 2015). These investigations have invariably led to the recognition that the economic and environmental performance of the cellulosic fuel industry can be improved through the selection of biomass substrates which require lower energetic and chemical inputs for their deconstruction (Torres et al. 2013; van der Weijde et al. 2013).
With a wealth of dedicated agronomic and genomic resources, advancing dual-purpose maize with improved biomass-processing amenability is a realistic prospect (Torres et al. 2014). Extensive evidence has demonstrated that maize conceals a considerable degree of genetic variation for cell wall compositional traits of beneficial value for bio-based industrial applications (Lorenz et al. 2009, 2010; Torres et al. 2015). These results suggest that favorable genetic gains for complex cell wall characteristics are attainable by exploiting available germplasm resources through classical breeding and selection. Despite these promising projections, nevertheless, much remains to be investigated in relation to how contrasting environments influence the expression of cell wall composition and bioconversion traits relevant to cellulosic fuel production, and how the latter are inherited in hybrid combinations. Certainly, this information will be deemed essential when designing selection strategies that maximize the efficacy of bio-based maize breeding endeavors.
This study was concerned with two distinct, yet inter-related objectives. The first one was to assess whether heritable variation (at the inbred level) for maize cell wall composition and degradability characteristics relevant to cellulosic fuel production remains stable across contrasting environments. The second one was to investigate how this variation, especially in relation to bioconversion traits, is inherited and expressed in hybrid combinations. Collectively, these analyses would yield insights into the technical feasibility of exploiting standing variation for complex maize cell wall characteristics at the inbred level for the production of superior hybrid cultivars with reduced lignocellulose recalcitrance and improved processing amenability. To this end, a panel of maize double haploid (DH) lines and their corresponding test-cross offspring were tested under different locations (primarily in the Netherlands) and characterized for a variety of cell wall compositional and bioconversion features relevant to cellulosic fuel production via dilute-acid hydrolysis and enzymatic saccharification.
Materials and methods
Plant material
A maize population of doubled haploids (DHs), property of Limagrain Nederland B.V. (Rilland, The Netherlands), was grown in 2009 at Wouw, The Netherlands, and was characterized for variation in cell wall composition and degradability traits relevant to cellulosic fuel production (Torres et al. 2015). The experimental population, consisting of 230 genotypes, was developed from the cross between proprietary inbred lines Lim-531 and Lim-789, highly differing in ruminal cell wall digestibility. From this trial, a panel of 34 DH genotypes (henceforth referred to as the DH-set) was selected to evenly represent the range of variation in cell wall bioconversion traits observed across the DH population. In parallel, these 34 lines were crossed to a Limagrain proprietary tester to produce a corresponding set of test-cross (TC) hybrids (henceforth referred to as the TC-set). The tester line was selected because of its favorable combining ability effects for cell wall digestibility traits in commercial test-cross procedures.
Field evaluations
The DH-set was employed to study the extent and stability of heritable variation for maize cell wall composition and bioconversion traits across contrasting environments. DH experiments were conducted during the summer of 2013 at three distinct locations: Steenbergen (The Netherlands), Wageningen (The Netherlands) and Greven (Germany). Trials were sown in replicate in adjacent randomized blocks. Genotypes were planted in two-row plots with a length of 1.5 m and an inter-row distance of 0.77 m at a density of 10 plants m−1. Per plot, stalks of 8 randomly selected plants were harvested at a 10 cm stubble height prior to silage maturity (between 6 and 8 weeks after the population’s mean silking period). Due to logistic constraints, however, test locations had to be harvested on separate dates (Table 1). Collected biomass feedstocks were chopped and air dried at 70 °C for 48 h, and were subsequently ground through a 1-mm screen using a hammer mill. Feedstock samples used from these trials (102 in total) were produced by pooling, per genotype, the milled material collected from two replicate experimental plots within each location (three locations).
The TC-set was used to investigate how genetic variation for maize cell wall traits is inherited and expressed in hybrid combinations. TC experiments were also conducted during the summer of 2013, but were sown in Eindhoven and Wouw (both in The Netherlands). The experimental design, harvesting methodology and sample processing procedures for TC experiments were identical to those prescribed for the DH trials; although in the TC trials, plants were harvested at silage maturity (Table 1).
Compositional analyses
Neutral detergent fiber (NDF), acid detergent fiber (ADF) and acid detergent lignin (ADL) components were determined through the ANKOM filter bag method (ANKOM Technology Corporation, Fairpoint, NY), which fundamentally derives from the work of Goering and Van Soest (1970). All analyses were performed in duplicate and were carried out using an ANKOM 2000 Fiber Analyzer (ANKOM Technology Corporation, Fairpoint, NY). The proportion of cellulose (Cel/CW), hemicellulose (Hem/CW) and acid-insoluble lignin (Lig/CW) contents on a cell wall basis were derived from detergent fiber data as described in Table 2.
The degree of substitution of cell wall glucuronoarabinoxylans (DHS), measured as the ratio of cell wall arabinose-to-xylose, was derived from the analysis of cell wall neutral sugar components; the latter determined by gas chromatography essentially as described by Englyst and Cummings (1984). Briefly, lyophilized water un-extractable solids were first treated with 72 % sulphuric acid (1 h, 30 °C), followed by a second hydrolysis process with 4 % sulphuric acid (3 h, 100 °C). Released neutral sugars were then derivatized to their respective alditol isoforms and quantified on an Agilent 7890A Gas Chromatography System (Agilent Technologies, Santa Clara, CA) using a DB-250 column (Agilent Technologies, Santa Clara, CA).
Bioconversion efficiency
Bioconversion efficiency following mild dilute-acid pretreatment and enzymatic hydrolysis was determined as detailed by Torres et al. (2013). In essence, biomass samples (500 mg) were pretreated at a 30 % solids loading in 0.17 % (w/v) sulfuric acid for 30 min at 140 °C. Subsequently, pretreated samples were treated with 250 µL of an Accelerase 1500 cellulolytic enzyme cocktail (Genencor B.V., Leiden, NL) in 40 mL 0.1 M citrate buffer. The enzyme load provided 50 filter paper units (FPU) of cellulase per gram cellulose. Samples were subsequently incubated at 50 °C in an Innova 42 air incubator (New Brunswick Scientific, Enfield, CT) at 200 RPM for 24 h. Saccharification liquors were analyzed for glucose concentration using a Boehringer Mannheim d-Glucose kit (Boehringer Mannheim, Indianapolis, IN, USA). The colorimetric assay was adapted to a 96 micro-titer plate format, and spectrophotometric reads were made using a Bio-Rad 550 Micro-plate Reader (Bio-Rad, Richmond, CA). For all samples, glucose content was expressed as both, the amount of glucose released from one gram of dry biomass (Glu-Rel) and the percentage of total cell wall glucose released upon enzymatic saccharification (Glu-Con) (Table 2).
Statistical analyses
DH and TC experiments were analyzed separately. Restricted Maximum Likelihood (REML) was used to determine the significance of genotypic and location differences in cell wall compositional and bioconversion traits. Using the GenStat for Windows 16th Edition Software Package (VSN International, Hemel Hempstead, UK), data were analyzed following the model defined by Holland et al. (2003) for the estimation of trait heritabilities from multiple environments with one replication per environment. The GenStat directive VCOMPONENTS was employed to specify a model in which location was treated as a fixed factor and genotype as a random factor. Subsequently, the REML directive was applied to obtain estimates of genotypic and phenotypic variances, which were ultimately used to calculate trait heritability estimates (h 2). Sources of variation were tested for significance by the Wald z-test.
Coefficients of genetic variation over genotype means (CVG) were also calculated for all evaluated traits and were used as standardized measures of genotypic variation. Inter-relationships between cell wall compositional and degradability traits within experiments, and between the performance of DH and TC lines for each evaluated trait, were analyzed by means of Pearson correlations over genotypic means. These statistical analyses were also performed using the GenStat for Windows 16th Edition Software Package (VSN International, Hemel Hempstead, UK).
Results and Discussion
Phenotypic variation for complex cell wall characteristics is highly heritable and stable across environments
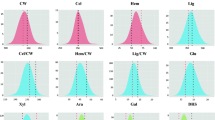
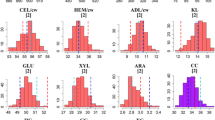
In this study, a panel of inter-related maize doubled-haploid (DH) genotypes was investigated across multiple locations for variation in cell wall composition and bioconversion properties relevant to cellulosic fuel production. Overall, the influence of location was highly significant (p < 0.001) for all investigated cell wall parameters, but the extent and pattern of fluctuations in cell wall compositional means across environments varied considerably from trait to trait (Fig. 1). To illustrate, average cellulose conversion efficiency for Greven (Glu-Con = ~49 %) was significantly higher than Glu-Con means reported for all other locations. In fact, at Steenbergen, Wageningen and Wouw, the DH-set displayed lower but fairly similar mean enzymatic convertibility rates (Glu-Con = ~41 %). By contrast, DH-set average values for lignin content (Lig) displayed broader variation across tested environments; this time, Greven ranked lowest (Lig = 12 g Kg−1 DM), but Steenbergen (Lig = 32 g Kg−1 DM) exhibited markedly higher values than Wageningen (Lig = 23 g Kg−1 DM) and Wouw (Lig = 27 g Kg−1 DM).
Box-plots summarizing the extent of variation of a panel of DH genotypes (34 in total) for diverse cell wall characteristics relevant to cellulosic fuel production. For every box-plot, horizontal solid lines represent DH-set medians, boxes represent the interquartile range and bars indicate extremes. In every panel, the x-axis indicates the location of DH trials, as specified in the legend
Differences in cell wall compositional profiles across environments can often be ascribed to management and harvesting practices leading to inter-location differences in plant maturity (Deinum and Struik 1989; Buxton 1996). The significantly lower lignin contents (Lig, Lig/CW) and improved conversion efficiencies (Glu-Con) reported for Greven relative to Steenbergen and Wageningen could be explained by the fact that harvesting occurred considerably earlier at this site (Table 1). As the maize plant matures, changes in the compositional balance of stem cell wall polymers lead to an increased concentration of phenolic components and a concomitant decrease in cell wall degradability properties (Jung and Casler 2006). Incidentally, mean lignin content (Lig, Lig/CW) was higher, and mean conversion efficiency (Glu-Con) was lower in Wouw, relative to Greven, even though both trials were harvested around the same period (albeit in different years). Seasonal and spatial variation in “environmental” conditions has also been shown to alter maize lignocellulose constitution and quality (Buxton 1996; Bruinenberg et al. 2002), and could be the main cause behind cell wall compositional differences observed for Greven and Wouw. Relevant factors affecting cell wall characteristics include temperature, light intensity and water availability. Given the substantial agro-climatic contrasts observed across our trials (Table 1), it seems plausible that systematic variation for cell wall polymeric profiles across locations is also a reflection of constitutive adaptations to divergent environmental conditions.
Regardless of the extent of environmental influences, highly significant (p < 0.001) genotypic differences were detected for all evaluated parameters. Means, descriptive statistics and narrow-sense heritability estimates (h 2) for the DH-set are summarized in Table 3. As expected, among cell wall components, variation was highest for Lig and Lig/CW (CV G = 16 %), but also for DHS (CV G = 8 %). These observations reinforce the notion that natural diversity in the biochemical composition of the maize cell wall and its physical properties is primarily ascribed to variation in the balance, monomeric make-up and ultra-structure of non-cellulosic cell wall polymers (Fontaine et al. 2003; Barriere et al. 2008; Torres et al. 2013, 2015). In this context and concurrent with previous studies (Lorenzana et al. 2010; Torres et al. 2013, 2015), correlation analyses confirm that the extent of enzymatic depolymerization (Glu-Con) of maize biomass is strongly, and negatively associated (r > −0.75) to the concentration of cell wall phenolics (Lig, Lig/CW), and positively impacted (r = 0.85) by increments in the degree of substitution (DHS) of cell wall glucuronoarabinoxylans. Correspondingly, across the DH-set, genotypic differences for cell wall bioconversion traits (Glu-Rel, Glu-Con) were likewise prominent (CV G = 9 %; in both cases). In particular, the maximal difference (min–max) for Glu-Con across lines was approximately 23 %; a finding that reiterates forage maize as a promising genetic resource for advancing complex biomass degradability properties in bioenergy-maize breeding programs (Torres et al. 2015).
Equally noteworthy, narrow-sense heritabilities (estimated across test locations) were high (h 2 > ~0.75) for the majority of investigated traits (Table 3), including the highly polygenic bioconversion characters Glu-Rel and Glu-Con. This observation is in agreement with prior findings emphasizing on the highly heritable nature of maize cell wall phenolic characteristics and ruminal cell wall digestibility properties across multi-location and multi-year trials (Dolstra et al. 1992; Méchin et al. 2001; Barriere et al. 2008; Lorenz et al. 2010; Lorenzana et al. 2010). In fact, Dolstra et al. (1992) had alluded that effective genetic gains for highly variable cell wall digestibility properties were theoretically possible without recurring to intensive replicated testing.
High heritabilities for quantitative cell wall traits observed here and elsewhere are the product of two factors: broad genetic diversity and the stability of genotypic differences across contrasting environments. In our investigation, pooling of samples did not allow estimation of G × E effects per se, but the stability in the span of cell wall trait ranges (Fig. 1) and the low frequency of genotype-rank cross-over events across environments (data not shown) suggest that the magnitude of G × E interactions was limited. Presumably, extensive heritable variation for the biochemical makeup and biological functionality of the maize cell wall is the result of adaptation to a wide range of agro-climatic conditions and end-uses. Contradictorily, despite being a highly complex and polygenic process (similar to “yield”), maize cell wall biosynthesis appears to adhere intractably to a pre-determined genetic blueprint (unlike “yield”) and appears rather impervious to genotype-by-environment (G × E) interaction effects (Argillier et al. 1996, 2000). It would appear as if maize cell wall construction (at the individual/genotype level) is under the control of a highly robust genetic system which constrains cell wall phenotypic plasticity, probably because a functional cell wall is crucial to plant fitness.
Doubled haploids and related hybrids display similar patterns cell wall architecture and degradability properties
Test-cross (TC) hybrids derived from our selection of DH genotypes were investigated across two locations; Eindhoven and Wouw. Trait means, ranges and broad-sense heritability estimates (h 2) for all investigated parameters are summarized in Table 4.
Significant genotypic differences (p < 0.05) were observed across the TC-set for all investigated cell wall characteristics, except for Hem/CW. As anticipated for TC hybrids sharing a common parent, trait ranges (min–max) in the TC-set were substantially lower than corresponding values observed for the DH-set (Hallauer et al. 2010). This held especially true for Hem/CW and Glu-Con, for which maximal differences across genotypes in the TC-set were reduced by nearly 50 %. Regardless of these noticeable reductions, variation among hybrids for most cell wall characteristics remained high. Once again, Lig, Lig/CW and DHS displayed the highest levels of genotypic variation (CV G > ~7 %). Likewise, Glu-Con was highly variable across the TC-set (CV G = 9 %), and we speculate that maximal differences (~11.0 %) could have been even larger, if the DH panel had included the population extremes. As was the case for the DH-set, cell wall trait broad-sense heritabilities were generally high (~0.60 < h 2 < ~0.80); thus reiterating our previous asseveration that phenotypic variation for complex cell wall characteristics is primarily influenced by additive genetic factors.
We have also detected systematic differences between DH lines and their TC offspring in the content and polymeric balance of cell wall polymers (Table 5). Specifically, hybrid genotypes displayed a greater accumulation of cell wall material (CW) in stem tissues and exhibited a higher proportion of lignin (Lig, Lig/CW) and cellulose (Cel/CW) in their cell walls. Presumably, these structural and constitutional adaptations would lead to improved stalk mechanical-strength; the latter deemed necessary to sustain the increased growth rates and yields typical of hybrid maize (Appenzeller et al. 2004; Ching et al. 2006). These compositional contrasts, however, did not appreciably alter the prevalent inter-relations that exist between cell wall compositional characters and biomass enzymatic convertibility. In the TC-set, Glu-Con was also strongly, and negatively associated (r > −0.75) to the concentration of cell wall phenolics (Lig, Lig/CW), and was positively impacted (r = 0.57) by DHS. Expectedly, given their higher concentration in lignin content (Lig, Lig/CW) and reduced DHS, Glu-Con values were on average lower for TC genotypes than for DH lines (Table 5). Furthermore, while a significant association between Glu-Con and CW was not detected in the DH panel, a negative relation (r = −0.56) between these two traits was detected at the TC level. By extension, we presume that the lower enzymatic convertibility (Glu-Con) observed for TC genotypes can also be attributed to the increased thickness of their secondary cell walls. In this study, bioconversion assays for the DH- and TC-set, employed identical processing conditions, including the same concentration of sulfuric acid per gram of dry biomass (5 % w/w) during thermochemical pretreatment. Therefore, because TC genotypes display a higher content of cell wall per dry gram of biomass, the concentration of acid in relation to cell wall content was lower for TC lines, thereby rendering the conversion process less effective (Torres et al. 2013). Ultimately, these results reinforce the notion that the efficient deconstruction of biomass (under cellulosic fuel conversion platforms) is greatly conditioned by its biochemical composition, especially under suboptimal processing regimes. Therefore, conclusions and projections regarding the efficiency of biomass-to-ethanol conversion systems should be constructed based on models that closely emulate conditions used in the industry. In this regard, since the lignocellulosic composition of hybrid maize differs from that of DH/inbred maize, different sets of analytical conditions should be employed to explore their bioconversion potential.
Test-cross performance for relevant cell wall characteristics can be predicted at the DH level
In our study, correlations between the performance of DH lines and related hybrids were significant and favorable for most investigated traits (Table 6). Strong associations (r > ~0.6) were especially prominent for Lig, Lig/CW and Glu-Con. Extensive evidence has demonstrated that genotypic differences for complex cell wall characteristics, which define or describe qualitative properties of maize lignocellulosic biomass (i.e. Lig, Lig/CW, DHS and Glu-Con), are independent of developmental variation (Dolstra and Medema 1990; Dolstra et al. 1992). Therefore, the correlated performance of DH lines and their corresponding TC offspring substantiates the notion that variation for complex cell wall characteristics is quantitatively inherited and predominantly additive. Only for CW, the observed positive association between DH and TC performances can be partially attributed to earliness effects.
Understandably, the lack of strict proportionality (r < 1) between DH per se and hybrid values is a consequence of the fact that genotypic means for DH and TC genotypes cannot be determined with complete accuracy (h 2 < 100 %). In addition, discrepancies between expected and realized hybrid performance might be partially attributed to the occurrence of non-additive gene action (e.g. dominance effects at heterozygous loci) in specific allelic combinations. Previous investigations have demonstrated, nevertheless, that for complex cell wall characteristics, genetic variation due to additive effects is generally more important than variation attributed to non-additive gene action (Deinum 1988; Deinum and Struik 1989; Argillier et al. 1996, 2000).
Ultimately, our observations suggest that preliminary selection for improved biomass composition and bioconversion properties at the DH level will guarantee similar correlated genetic gains in hybrid breeding. Moreover, because biomass processing amenability (Glu-Rel, Glu-Con) is intrinsically defined by the chemical constitution of plant cell walls, hybrid performance for bioconversion traits could be theoretically predicted by models which account for genetic variation in multiple cell wall characteristics. Correspondingly, we have idealized a regression model (described in Table 6) to forecast Glu-Con values at the hybrid level based on the performance of DH lines for CW, Lig and DHS; all shown to impact the extent of maize biomass convertibility. Notably, predicted values correlated strongly (r = 0.78) with realized means and effectively surpassed the predictive accuracy (r = 0.60) of the DH-TC correlated response for Glu-Con. From a commercial standpoint, the ability to predict hybrid performance based on the productivity of parental lines offers important practical, technical and economic advantages. On the one side, effective selection at the DH/inbred level will prove highly advantageous if fewer resources are devoted to factorial test-cross procedures and evaluations. On the other hand, the dissection of complex biomass quality traits may lead to the identification of phenotypic attributes which can be measured with greater precision and at a lower cost. Generally, these component traits display simpler inheritance patterns and their targeted selection can yield greater genetic gains with respect to highly complex cell wall quality traits.
Conclusions
The results of this investigation prescribe positive prospects and practical advantages for the development of bioenergy maize cultivars with improved cell wall characteristics. In particular, the high heritability of cell wall compositional and degradability properties (ascribed primarily to broad genetic diversity and the stability of genotypic differences for these traits across environments representative for NW Europe) guarantee high selection efficacy during the development of superior DH/inbred material, and predispose that multi-environment testing will only be necessary at advanced stages of bioenergy-maize breeding programs. Moreover, because genetic variation for complex cell wall characteristics is predominantly additive, preliminary selection at the inbred level will expectedly lead to successful hybrid selection; thereby minimizing the need for recurrent test-crossing procedures and evaluations. Notwithstanding, because inbred and hybrid maize exhibit seemingly distinct cell wall compositional profiles, careful consideration is required when determining optimal analytical parameters for evaluating their bioconversion potential.
Cell wall bioconversion traits (Glu-Con, Glu-Rel) constitute accessible and reliable selection criteria which can be incorporated in modern breeding programs seeking to develop advanced bio-based hybrid varieties. And while the convergence of classical selection schemes with advanced marker-assisted selection strategies (e.g. genomic selection) can accelerate maize cultivar development for bioenergy applications, maximal genetic gains are expected from breeding programs focusing on preselected germplasm harboring substantial levels of favorable genotypic variation for relevant target traits. In this respect, we advocate the screening of elite forage maize germplasm known to display substantial amounts of genetic variability for biomass yield, cell wall composition and cell wall degradability properties relevant to cellulosic fuel production.
References
Appenzeller L, Doblin M, Barreiro R, Wang H, Niu X, Kollipara K, Carrigan L, Tomes D, Chapman M, Dhugga KS (2004) Cellulose synthesis in maize: isolation and expression analysis of the cellulose synthase (CesA) gene family. Cellulose 11:287–299
Argillier O, Barrière Y, Lila M, Jeanneteau F, Gélinet K, Ménanteau V (1996) Genotypic variation in phenolic components of cell-walls in relation to the digestibility of maize stalks. Agronomie 16:123–130
Argillier O, Méchin V, Barrière Y (2000) Inbred line evaluation and breeding for digestibility-related traits in forage maize. Crop Sci 40:1596–1600
Barrière Y, Thomas J, Denoue D (2008) QTL mapping for lignin content, lignin monomeric composition, p-hydroxycinnamate content, and cell wall digestibility in the maize recombinant inbred line progeny F838 × F286. Plant Sci 175:585–595
Bruinenberg M, Valk H, Korevaar H, Struik P (2002) Factors affecting digestibility of temperate forages from seminatural grasslands: a review. Grass Forage Sci 57:292–301
Buxton DR (1996) Quality-related characteristics of forages as influenced by plant environment and agronomic factors. Anim Feed Sci Tech 59:37–49
Ching A, Dhugga KS, Appenzeller L, Meeley R, Bourett TM, Howard RJ, Rafalski A (2006) Brittle stalk 2 encodes a putative glycosylphosphatidylinositol-anchored protein that affects mechanical strength of maize tissues by altering the composition and structure of secondary cell walls. Planta 224:1174–1184
Deinum B (1988) Genetic and environmental variation in quality of forage maize in Europe. Neth J Agr Sci 36:400–403
Deinum B, Struik P (1989) Genetic variation in digestibility of forage maize (Zea mays L.) and its estimation by near infrared reflectance spectroscopy (NIRS). An analysis. Euphytica 42:89–98
Dien B, Sarath G, Pedersen J, Sattler S, Chen H, Funnell-Harris D, Nichols N, Cotta M (2009) Improved sugar conversion and ethanol yield for forage sorghum (Sorghum bicolor L. Moench) lines with reduced lignin contents. BioEnergy Res 2:153–164
Dolstra O, Medema J (1990) An effective screening method for genetic improvement of cell-wall digestibility in forage maize. In: Proceedings 15th congress maize and sorghum section of Eucarpia, pp 4–8
Dolstra O, Medema J, De Jong A (1992) Genetic improvement of cell-wall digestibility in forage maize (Zea mays L.). I. Performance of inbred lines and related hybrids. Euphytica 65:187–194
Englyst HN, Cummings JH (1984) Simplified method for the measurement of total non-starch polysaccharides by gas-liquid chromatography of constituent sugars as alditol acetates. Analyst 109:937–942
Fontaine A-S, Bout S, Barrière Y, Vermerris W (2003) Variation in cell wall composition among forage maize (Zea mays L.) inbred lines and its impact on digestibility: analysis of neutral detergent fiber composition by pyrolysis-gas chromatography-mass spectrometry. J Agr Food Chem 51:8080–8087
Fu C, Mielenz JR, Xiao X, Ge Y, Hamilton CY, Rodriguez M, Chen F, Foston M, Ragauskas A, Bouton J, Dixon RA, Wang Z-Y (2011) Genetic manipulation of lignin reduces recalcitrance and improves ethanol production from switchgrass. P Natl Acad Sci USA 108:3803–3808
Goering H, Van Soest PJ (1970) Forage fiber analyses (apparatus, reagents, procedures, and some applications), vol 379. US Agricultural Research Service, Washington, DC
Hallauer AR, Carena MJ, Miranda Filho JD (2010) Quantitative genetics in maize breeding. Springer, Dordrecht
Holland JB, Nyquist WE, Cervantes-Martínez CT (2003) Estimating an interpreting heritability for plant breeding: an update. Plant Breed Rev 22:9–11
Jung HG, Casler MD (2006) Maize stem tissues: impact of development on cell wall degradability. Crop Sci 46:1801–1809
Lorenz AJ, Coors JG, de Leon N, Wolfrum EJ, Hames BR, Sluiter AD, Weimer PJ (2009) Characterization, genetic variation, and combining ability of maize traits relevant to the production of cellulosic ethanol. Crop Sci 49:85–98
Lorenz A, Coors J, Hansey C, Kaeppler S, De Leon N (2010) Genetic analysis of cell wall traits relevant to cellulosic ethanol production in maize (Zea mays L.). Crop Sci 50:842–852
Lorenzana RE, Lewis MF, Jung HJG, Bernardo R (2010) Quantitative trait loci and trait correlations for maize stover cell wall composition and glucose release for cellulosic ethanol. Crop Sci 50:541–555
Méchin V, Argillier O, Hébert Y, Guingo E, Moreau L, Charcosset A, Barrière Y (2001) Genetic analysis and QTL mapping of cell wall digestibility and lignification in silage maize. Crop Sci 41:690–697
Torres AF, van der Weijde T, Dolstra O, Visser RGF, Trindade LM (2013) Effect of maize biomass composition on the optimization of dilute-acid pretreatments and enzymatic saccharification. BioEnergy Res 6:1038–1051
Torres AF, Visser RG, Trindade LM (2014) Bioethanol from maize cell walls: genes, molecular tools and breeding prospects. GCB Bioenergy 1:17. doi:10.1111/gcbb.12164
Torres AF, Noordam-Boot CMM, Dolstra O, van der Weijde T, Combes E, Dufour P, Vlaswinkel L, Visser RGF, Trindade LM (2015) Cell wall diversity in forage maize: genetic complexity and bioenergy potential. BioEnergy Res 8:187–202
van der Weijde T, Alvim Kamei CL, Torres AF, Vermerris W, Dolstra O, Visser RGF, Trindade LM (2013) The potential of C4 grasses for cellulosic biofuel production. Front Plant Sci 4:1–18
Acknowledgments
We gratefully acknowledge Genencor International B.V. for kindly supplying us with their cellulolytic enzyme cocktails used in this study. Within the framework of the Carbohydrate Competence Centre, this research has been financially supported by the European Union, the European Regional Development Fund, and the Northern Netherlands Provinces (Samenwerkingsverband Noord-Nederland), KOERS NOORD.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Torres, A.F., Noordam-Boot, C.M.M., Dolstra, O. et al. Extent of genotypic variation for maize cell wall bioconversion traits across environments and among hybrid combinations. Euphytica 206, 501–511 (2015). https://doi.org/10.1007/s10681-015-1517-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-015-1517-x