Abstract
Purpose
We investigated whether group-level bias of a 24-h recall estimate of protein and potassium intake, as compared to biomarkers, varied across European centers and whether this was influenced by characteristics of individuals or centers.
Methods
The combined data from EFCOVAL and EPIC studies included 14 centers from 9 countries (n = 1,841). Dietary data were collected using a computerized 24-h recall (EPIC-Soft). Nitrogen and potassium in 24-h urine collections were used as reference method. Multilevel linear regression analysis was performed, including individual-level (e.g., BMI) and center-level (e.g., food pattern index) variables.
Results
For protein intake, no between-center variation in bias was observed in men while it was 5.7% in women. For potassium intake, the between-center variation in bias was 8.9% in men and null in women. BMI was an important factor influencing the biases across centers (p < 0.01 in all analyses). In addition, mode of administration (p = 0.06 in women) and day of the week (p = 0.03 in men and p = 0.06 in women) may have influenced the bias in protein intake across centers. After inclusion of these individual variables, between-center variation in bias in protein intake disappeared for women, whereas for potassium, it increased slightly in men (to 9.5%). Center-level variables did not influence the results.
Conclusion
The results suggest that group-level bias in protein and potassium (for women) collected with 24-h recalls does not vary across centers and to a certain extent varies for potassium in men. BMI and study design aspects, rather than center-level characteristics, affected the biases across centers.
Similar content being viewed by others
Introduction
There is an increasing interest in identifying and understanding geographical variations in dietary intake. For instance, a number of international studies have been performed in Europe with the purpose of investigating dietary exposure and testing hypotheses on diet–disease associations assessing dietary intake collected in different geographical areas [1–3]. Another example is that dietary intake data collected through national nutritional monitoring surveys across different European countries can be used to develop and evaluate nutritional policies under the EU commission framework [4]. However, to correctly estimate the variation in dietary intake across populations in those investigations, it is necessary to obtain data that are as accurate and comparable as possible.
The collection of dietary data for comparisons between populations should preferably be performed using the same and standardized dietary assessment method. To that end, a repeated nonconsecutive 24-h dietary recall interview using EPIC-Soft has been recommended for assessing dietary intake in future national food consumption surveys [4, 5]. Subsequently, the evaluation of this method was performed within the European Food Consumption Validation (EFCOVAL) study [6] .
An established approach to evaluate the validity of dietary assessment instruments is to compare self-reported dietary intake with its related biomarker estimates. In particular, recovery-based biomarkers have a precisely known quantitative relation to absolute daily intake and are a valid reference to estimate the bias in dietary intake reports [7]. Moreover, recovery biomarkers provide reference estimates of dietary intake with errors that are likely to be uncorrelated with the errors of self-reported dietary methods [8, 9]. Two of the few available recovery biomarkers to assess the bias in nutrient intake are urinary nitrogen and potassium [10, 11].
Previously, the accuracy of protein as estimated by one 24-h dietary recall using EPIC-Soft has been evaluated using urinary nitrogen in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. In this study, protein intake was underestimated at the group level and varied across European centers, that is, ratios between nitrogen intake and excretion ranged from 0.69 (Greece) to 0.99 (Ragusa-Italy) in men and from 0.54 (Greece) to 0.92 (Paris-France) in women [12]. More recently, in the EFCOVAL study, the average of two nonconsecutive days of protein and potassium intake assessed with this computerized 24-h recall and compared to their respective biomarkers revealed underestimation that ranged across five European centers between 2 and 13% for protein intake and between 4 and 17% for potassium intake [13]. These results suggested that differences in the performance of the 24-h recall may exist across European countries.
A number of reasons have been hypothesized to explain the observed variation in biases in protein and potassium intake between-centers in the EPIC and EFCOVAL studies. For instance, differences in characteristics at the center (e.g., food pattern) or individual level (e.g., socioeconomic status, BMI) could explain differential misreporting of dietary intake. However, an evaluation of the potential effect of characteristics at the individual and center (country) level on the validity of the method was lacking. The analyses initially conducted in the EPIC and EFCOVAL data on protein and potassium bias used a single-level model with ‘fixed effects’, which did not allow for simultaneous separation of within- and between-center variance. These previous analyses also did not consider all possible explanatory variables at the individual and center levels to be included in the model. Therefore, to gain a more in-depth understanding of the accuracy of nutrient intake assessed by the 24-h recall across European centers, the individual and center level ought to be considered simultaneously. For that purpose, multilevel modeling can be used by means of ‘random effect models’. The random effect model approach allows for estimating the effects of individual- and center-level characteristics, and their impact on the estimates of between-center variation in the bias of nutritional assessment [14].
Furthermore, pooling the data from the EFCOVAL and EPIC studies increased the number of geographical regions considered, the heterogeneity of the dietary patterns and the statistical power to evaluate the bias in protein and potassium intake collected with 24-h recalls across European populations using multilevel analysis. Therefore, the objective of this paper was to further investigate whether the group-level bias in intake of protein and potassium collected with 24-h recalls using EPIC-Soft varied across European centers and whether this was affected by characteristics at the individual and center level.
Subjects and methods
Study population
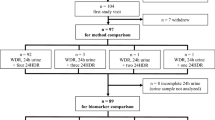
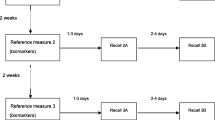
This study combines study populations from two European studies, the EPIC calibration sub-study and the EFCOVAL validation study, together representing 9 European countries. Previous publications described in detail the rationale and methods of the studies [1, 15–17]. Within the EPIC cohort, ~37,000 individuals comprised the subsample of the calibration sub-study. Between 1995 and 2000, these individuals were randomly chosen from the EPIC cohorts for completing a single standardized 24-h dietary recall (EPIC-Soft) to calibrate baseline food frequency questionnaires (FFQ) [1]. More details about the study population from the calibration study are reported elsewhere [9, 12]. In a convenience subsample of the calibration study, 24-h urines were collected from 1,386 participants from 12 EPIC centers in 6 countries (Paris in France; Florence, Naples, Ragusa, Varese and Turin in Italy; some combined regions in Greece; Cambridge and Oxford in the United Kingdom; Bilthoven in the Netherlands; and Heidelberg and Potsdam in Germany). Urine was collected over the same day as the 24-h recall (44%) or within a maximum of 6 days afterward (56%). Furthermore, lifestyle information was collected at baseline from all EPIC study participants. To optimize the sample sizes in some centers, the initial 12 centers from the EPIC administrative areas were redefined into 9 centers [12], labeled hereafter as Heidelberg, Potsdam, Paris, Greece, Central/Southern Italy (including Florence, Naples and Ragusa), Northern Italy (including Varese and Turin), Bilthoven, Cambridge, and Oxford. In the EFCOVAL validation study, dietary information was collected in five European centers, that is, Ghent (Belgium), Brno (Czech Republic), Nice (France), Wageningen (the Netherlands) and Oslo (Norway), in the years 2007 and 2008. In total, 600 participants underwent two standardized 24-h recall interviews using EPIC-Soft software and following a randomized schedule [13]. In addition, they provided two 24-h urines, covering the same days as the 24-h recalls. Participants were healthy individuals, who did not take diuretics or followed prescribed therapy. Both studies were conducted according to the guidelines laid down in the Declaration of Helsinki, and procedures involving human subjects were approved by ethical committees of the centers involved in the data collection.
In the combined assessment, data from 1986 participants from 14 European centers (9 from the EPIC study) were initially used. From these, 145 participants were excluded from the protein analyses and 176 from the potassium analyses. Reasons for exclusion were that data of the 24-h recall (n = 18), urinary protein (n = 13) or potassium (n = 44) was not available, participants were on a restricted diet (n = 51), or <50% of para-aminobenzoic acid (PABA) was recovered (n = 63)—see details in the 24-h urine collection section. Thus, the final sample in the data set included 1,841 participants for the data analyses of protein and 1,810 for potassium.
An overview of the two studies and the pooled data are given in Table 1.
Dietary data
In both the EPIC and the EFCOVAL study, the 24-h recalls were collected using EPIC-Soft version 9.16. The structure and standardization procedure of EPIC-Soft have been described elsewhere [13, 18, 19]. Briefly, EPIC-Soft is a computer-assisted 24-h dietary recall that follows standardized steps when describing, quantifying, probing and calculating dietary intake [18]. The 24-h recalls were collected by trained dietitians through face-to-face interviews in the EPIC centers. In EFCOVAL, one telephone and one face-to-face interview were applied in random order in each subject. These were also applied by trained dietitians or nutritionists who followed a course using similar instructions and guidelines as used in the EPIC study. In both studies, dietary data of all days of the week were collected. In EPIC, protein and potassium food composition values from each national food composition database were standardized across countries within the European Nutrient Database (ENDB) project, in collaboration with national compilers and other international experts [20]. For EFCOVAL, protein and potassium intake were calculated using country-specific food composition databases.
To align both EFCOVAL and EPIC data sets, only the first 24-h recall information from the EFCOVAL participants was pooled in the data set. Consequently, the EFCOVAL measurements consisted of 24-h recalls collected by telephone and face-to-face interviews. Furthermore, an attempt has been made to standardize food composition values between EPIC and EFCOVAL studies. Similar to what has been done within the ENDB framework, losses in the potassium values of cooked single foods, that have been linked to raw foods in the food composition data, were adjusted by applying the same retention factors than those initially used for the EPIC data. For protein, standardization of the EPIC data was performed by applying the 6.25 conversion factor (CF) instead of food-specific CFs to convert nitrogen into protein intake. Within EFCOVAL, such standardization was only possible for the data from Wageningen (NL) and Nice (FR) because it was not possible to retrieve the original CF information applied in the protein composition of the foods in the other three centers that represented 9.9% of the total study population. Energy values were computed by adding the contributions from protein, carbohydrates, fat and alcohol intake and using related Atwater factors (17, 17, 37 and 29 kJ per gram, respectively) [21].
There were some differences between the databases used in the EPIC-Soft software in the EPIC and EFCOVAL studies. These differences were mainly related to the upgrade of food lists, standard units, descriptors for food identification [22] and selection of food pictures for food quantification. Nevertheless, the purpose of updating these databases in EPIC-Soft was to take into account actual differences in consumption between the centers while the procedures to collect them were still standardized.
24-h urine collection and recovery biomarkers for protein and potassium intake
For the EFCOVAL participants, only the first 24-h urine collection corresponding to the first 24-h recall was used in the pooled data set. Twenty-four hour urine collections were verified for completeness by using para-aminobenzoic acid (PABA) tablets (PABAcheck, Laboratories for Applied Biology, London). Complete logistics of 24-h urine collections and laboratory analyses are described elsewhere [12, 13]. In brief, after collection, the 24-h urines were transported to the study centers where they were weighed and aliquoted. Then, specimens were stored at −20 °C until shipment on dry ice to the central laboratories in Cambridge (EPIC) and Wageningen (EFCOVAL). Urinary nitrogen was determined by the Kjeldahl technique in both studies. Urinary potassium was determined using an IL 943 flame photometer (Instrumentation Laboratory) in EPIC and using an ion-selective electrode on a Beckman Synchron LX20 analyzer in EFCOVAL. PABA was measured by colorimetry in both studies [12, 13, 23]. Urine samples with PABA recoveries below 50% were treated as incomplete and excluded from the data analyses. Specimens containing between 50 and 85% of PABA recovery had their urinary protein and potassium concentrations proportionally adjusted to 93% [24]. Furthermore, we did not exclude participants with PABA recovery above 110%, as we assumed that those collections were complete. This procedure for dealing with PABA recovery is different from previously published data in the EPIC study [12], resulting in a larger sample sizes for some EPIC centers. Taking into account extra-renal losses (~19%) and the fact that protein on average contains 16% of nitrogen, urinary protein was calculated as [6.25 × (urinary nitrogen/0.81)] [11, 25]. Urinary potassium was estimated by dividing the measured value by 0.77, assuming that 77% of potassium intake is excreted through the urine when considering fecal excretion [10, 26].
Laboratory calibration study
With the purpose of harmonizing biomarker laboratory data, a calibration study was conducted among laboratories that performed analyses in the EPIC and EFCOVAL studies. Therefore, during the Summer of 2008, 50 urine samples of the EPIC study that were previously analyzed for protein and potassium content by the MRC Dunn Clinical Nutrition Centre in Cambridge (UK) were reanalyzed by the laboratory at Wageningen University (NL). The results obtained from the two laboratories were compared. In addition, comparability of laboratory methods used in EPIC and EFCOVAL laboratories was further substantiated by evaluating standard reference materials and quality control procedures (e.g., inter-laboratory proficiency tests) of each laboratory measurement. A report of the laboratory comparison between studies is presented in the supplemental material online. Shortly, we did not observe statistically significant differences between the measurements by the two labs for nitrogen or potassium. Therefore, calibration of data between both studies was not necessary, and original biomarker data of the two studies were used in our analyses.
Anthropometrics and educational level
In both studies, measurements of body weight and height were collected for the calculation of body mass index (BMI). In EPIC, some measurements were self-reported and were corrected by prediction equations, as described in Haftenberger et al. [27].
Furthermore, a general lifestyle questionnaire, including educational level information, was applied at the start of each study. Educational level was categorized using different categories in the EPIC and EFCOVAL studies (see Table 1). The proposed classification for the pooled data analyses included the following categories: none, low, intermediate and high, in which technical and secondary groups of education from the EPIC data were treated as intermediate levels.
Explanatory variables
Based on preexisting knowledge, we selected full sets of explanatory variables to be included in the models, which we expected to vary across individuals or centers and be correlated with the nutrient bias or intake or biomarker levels. Variables at the individual level were age (in years), educational level (categorical), BMI (in kg/m2), mode of administration of the 24-h recall (face-to-face vs. telephone), day of the week of the 24-h recall (weekday vs. weekend) and year of recruitment. Explanatory variables at center level were study (EFCOVAL vs. EPIC), human development index (HDI, [28]) and a food pattern index. The variable ‘study’ is meant to represent the distinct characteristics of each study, such as the period of data collection. We used the HDI as a proxy for identifying socioeconomic differences across the centers. The HDI statistic is composed from national data on life expectancy, education and per capita gross domestic product, as an indicator of standard of living, at the country level. Thus, centers in the same country had the same HDI. To capture the variability existing in food pattern across the European centers, a food pattern index was calculated for each individual and averaged out for each center. For this purpose, we used the variety index component, obtained from the ‘diet quality index-international’ (DQI-I)[29], to indicate the diversity in food group intake between the centers. This index assesses whether intake comes from diverse sources both across and within food groups and varies from 0 to 20 points. It is divided in two parts. First, the overall food group variety is assessed by inclusion of at least one serving food per day from each of the five food groups (meat/poultry/fish/egg, dairy, grains, fruits and vegetables). Second, variety within protein sources is evaluated, that is, number of protein sources. The lowest food index score in our assessment was attributed to Oxford (vegetarians)-UK (10.5 points) and the highest to the 3 Spanish centers (>18.5 points).
Statistical analyses
Multilevel linear regression models were used to assess the variation in group-level bias of protein and potassium intake across the centers and to estimate the effects of individual- and center-level explanatory variables on this variation. Individuals were set at the first level and centers at the second. Statistical analyses were conducted separately for men and women since our previous single-level analyses showed different group-level bias for each gender [13]. The number of centers in the analysis of each gender is different since the research center in Paris only included women.
Bias was defined as the ratio between nutrient intake and its excretion. We chose the ratios instead of absolute values to take into account differences that were related to high or low protein and potassium intake across centers. These ratios were treated as the dependent variable in the regression models and were log-transformed to improve normality (ln(individual ratio)).
We fitted three regression models in an increasing order of complexity (see “Appendix”). Model (i) included a random effect to model between-center variation of protein and potassium biases across centers (i.e., random intercepts) without explanatory variables. Therefore, we were able to estimate the between-center variances in group-level bias in a crude model. In model (ii), individual-level explanatory variables were added to the fixed part of the model, whereas in model (iii), center-level variables were also included. Full sets of individual- and center-level explanatory variables were included in their respective regression models, and the optimal subsets of variables were chosen by using a backward selection. The fit of the models was tested by the likelihood ratio test, which compared minus twice the difference of the maximum likelihood (ML) of that model with the preceding nested model [14]. The likelihood ratio test statistic was compared to a χ2 distribution with degrees of freedom equal to the number of extra-parameters in the more complex model [14]. Results are only presented for models that showed a statistically significant improvement. Furthermore, we also attempted to include random slopes to allow the effects of age and BMI to vary between centers, but their results suggested homogeneity of the effects and they are, therefore, not included in the paper.
The total variance of log-transformed bias of each model was partitioned in two components, the between-center variance (or center random effect—\( \sigma_{u0}^{2} \)) and the within-center between-individual variance (or individual random effect—\( \sigma_{e0}^{2} \)). To quantify the variation in nutrient biases across centers, we looked at the between-center random effect obtained across the fitted models. Even though zero between-center variation in bias may have been observed in a simpler model, we proceeded with the more complex ones to check whether the variance estimates would change by including different terms into the model (e.g., inclusion of explanatory variables). To interpret the contribution of between-center variance, we used two approaches, the variance partition coefficient (VPC) and the coefficient of variation (CV) between centers. The VPC was calculated as the proportion of total variance that is due to differences between centers [14].
The CV expresses the variation in the bias between centers as a percentage, relative to the intake according to the reference method. Because the analysis of the bias was done on the logarithmic scale and the ratios on the center level were close to one,
Statistical analyses were carried out using SAS statistical package, version 9.1 (SAS Institute Inc., Cary, NC, USA).
Results
All centers combined, both men and women underreported protein intake from 1-day 24-h recall by 3 and 5% (ratio intake/excretion = 0.97 and 0.95), respectively (Table 2). In men, the ratio between protein intake and excretion varied from 0.89 in Wageningen (NL) to 1.03 in Central/Southern Italy (IT). In women, the ratio varied from 0.84 in Greece (GR) to 1.05 in Oslo (NO). Average underestimation of potassium intake was 1% in men and 3% in women. In men, the lowest ratio between potassium intake and biomarker excretion was observed in Nice (FR) and Heidelberg (DE) with 0.86, whereas the highest ratio was seen in Northern Italy (IT) with 1.17. In women, the lowest ratio was 0.90 in Potsdam (DE) and the highest ratio was 1.08 in Greece (GR).
Protein intake
Based on the center random effect, between-center variance in protein bias was null (\( \sigma_{u0}^{2} \) ~ 0) in men (Table 3). In women, the between-center CV in protein biases was initially 5.7%, which was 3% of the total variance, and Greece (GR), Paris (FR) and Oslo (NO) were the centers with a group-level bias deviating from the overall mean bias (Table 4). After inclusion of individual explanatory variables, especially BMI, the between-center variance in bias was reduced by 78% (from 0.0032 to 0.0007) in women (p < 0.001). In addition, the remaining between-center variance in protein biases (CV = 2.6%) was not significant anymore and no center appeared to deviate from the mean bias. Other variables that may have contributed to the reduction of between-center variance in protein biases in women were ‘day of the week’ (p = 0.06) and ‘mode of administration’ (p = 0.06). When we added center-level variables (e.g., HDI), we did not observe a significant improvement of the model’s fit neither for men nor women (data not shown). Therefore, model ii (random intercepts to model the center effect with inclusion of variables at the individual level) was retained as the most adequate model to the data (Tables 3 and 4).
Potassium intake
In men, the between-center CV in potassium biases was initially 8.9% (Model i), which was about 5% of the total variance (Table 5). When applying model ii, the between-center CV slightly increased to 9.5%. Furthermore, the biases from 4 centers, that is, Greece (GR), Heidelberg (DE), Nice (FR) and Northern Italy (IT), seemed to differ from the overall mean potassium bias. Individual BMI was a factor influencing the between-center variance in men (p = 0.002). No between-center variance (\( \sigma_{u0}^{2} \) = 0) was initially observed in the potassium biases in women (Table 6). After including individual variables in the model, BMI predicted the bias and there was still no significant variation across centers in women (CV = 1.7%). As for the protein analyses, inclusion of center-level variables (model iii) did not improve the fit of the model, for men and women.
Discussion
In this paper, we investigated the variation in group-level bias in self-reported protein and potassium intake collected with the computerized 24-h recall (EPIC-Soft) across European adult populations. By using a multilevel modeling approach, we observed that the bias in protein intake did not vary across centers in men, but varied among women (5.7% of variation) in the crude model with random intercepts. Bias in potassium intake differed between centers in men (8.9% of variation), but not in women. Explanatory variables at the individual level (i.e., BMI, day of the week and mode of administration) predicted and explained the between-center variation of bias in protein and potassium intake. When those were included in the model, the bias in protein intake in women did not significantly vary anymore, and the bias in potassium intake remained with variations across centers (9.5% of variation). Selected center-level variables (i.e., HDI) did not influence the between-center variations in bias in our assessment.
The major advantage of using multilevel analysis was that we were able to separate the two variance components (i.e., within- and between-center) in protein and potassium bias in one sole model, which is important for a reliable comparison of populations [30, 31]. In addition, in this unique setting of combining data sets from two European studies, we were able to use dietary and biomarker meas urements that were collected using standardized methodologies. A comparison of laboratory measurements was performed to overcome possible inter-laboratory errors, and an important level of standardization was achieved by estimating protein and potassium intake from food composition tables across the different European centers, although not completely for Ghent (BE), Brno (CZ) and Oslo (NO). Furthermore, the large number of centers originating from different regions of Europe allowed us to compare populations with different dietary intake profiles.
Yet, our study has limitations that should be considered in the interpretation of our findings and in the development of future research. First, we cannot assume that these results can be extrapolated for other points of the distribution of protein and potassium intake, which are important to assess prevalence above or below a certain cut off point [32]. As previously shown, we may expect that the accuracy of other points of the distribution, between the mean and the ends of the tails, is inferior compared to the mean bias at the population level [13]. Nevertheless, this has been the first attempt of using a multilevel approach to validate dietary intake in an international context, and an important understanding of between-center variation in nutrient intake bias as well as factors that can influence the performance of the method has been achieved. Second, we were not able to completely harmonize the food composition data for protein in EFCOVAL. However, when we excluded centers with nonstandardized protein composition data from our main analysis, the results for protein did not change. Third, it can be questioned whether we have properly dealt with the results of the laboratory comparison, considering the small sample size in the calibration study. Based on the nonstatistically significant differences obtained with the t test, we opted not to calibrate the laboratory estimates. However, multilevel analysis with and without calibration of protein and potassium biomarker values resulted in similar results. At last, the generalization of these results to other nutrients is not warranted given that foods and related nutrients might be differently misreported [33–35].
In other analysis with EFCOVAL and EPIC data [12, 13], the group-level bias of protein and potassium intake assessed with 24-h recalls varied across centers. A number of reasons were suggested to explain this variation in bias, as for instance a difference in BMI. Differential underreporting of dietary intake by overweight and obese individuals is expected based on the literature [36, 37]. Indeed, BMI was the explanatory variable predicting most of the bias in protein and potassium intake in this analysis as well as explaining the variation of bias across the centers; thus, confirming the importance of considering BMI when performing the 24-h recalls in Europe.
Besides BMI, the day of the week (weekday vs. weekends) and the mode of administration (face-to-face vs. telephone) appeared to influence the bias in protein intake across centers, but not in potassium. An explanation for this difference may be that potassium is a nutrient present in a greater variety of foods/food groups and more equally distributed among different food groups than protein [10]. Moreover, higher protein intake has been observed during weekends across European populations when compared to weekdays [38]. What regards the comparability of different modes of administration, comparable results between telephone and face-to-face interviews could be expected [39–41], but perhaps populations with different dietary intake patterns respond differently to these two modes of administration. Actually, within the EFCOVAL study, we observed that 24-h recalls collected by telephone interviews seemed to provide a more accurate assessment than by face-to-face interviews in some research centers (unpublished results).
Furthermore, we observed a between-center variation in group-level bias in potassium intake in men, but not in women. As differential reporting bias is suggested among genders, we speculate that improvements of the reported 24-h recalls might be expected if the person who does the shopping and/or the cooking of the foods is involved in the dietary interview.
We hypothesized that certain center characteristics (e.g., food pattern index, HDI) could influence the variation of group-level biases in protein and potassium intake across the European centers. However, we observed almost no variation in biases across the centers, except for bias in potassium intake in men. Therefore, there was not much variation in bias to be explained by characteristics at the center level. Nevertheless, we suppose that these characteristics may be relevant in the assessment of less regularly consumed nutrients and, especially, for foods and food groups, as we may expect a larger variation in the dietary intake assessment between populations in Europe than was found for the nutrients we assessed [42]. For that, more insight into food pattern indexes to represent country differences would be valuable, as the index we have used in this assessment may have not been sufficiently accurate.
Furthermore, the integration of the two study populations, which have dietary data collected in different time periods, did not seem to influence the variation in bias in protein and potassium intake across centers. Although slightly higher protein intakes have been observed in the EFCOVAL centers when compared to EPIC, neither the ‘year of recruitment’ nor the ‘period of collection’ (i.e., center-level variable: study) influenced the variation in bias. In addition, energy intake that was also slightly higher in the EFCOVAL study did not change any of the results when added as co-variable (results not shown). Only the fact that two modes of administrations were used in EFCOVAL, while only one was used in EPIC probably played a role in the difference in protein intakes across the two studies. In fact, mode of administration appeared to be significantly associated with the variation in bias in protein intake across the centers.
In conclusion, the present results appear to bring us a step further to understand and quantify the variation in bias in the assessment of protein and potassium intake collected with 24-h recalls across European centers. Remarkably, almost no variation in protein and potassium biases of the 24-h recalls using EPIC-Soft was observed across the centers. In addition, the results of this study suggest that the group-level bias in protein intake for both genders and potassium intake for women did not vary across centers and to a certain extent varied for potassium intake in men. Furthermore, the large number of centers originating from different regions of Europe allowed us to compare populations with different dietary intake profiles. In view of that, the data to be collected in future pan-European nutritional monitoring surveys should be analyzed and interpreted taking into account the characteristics that may influence reports of protein and potassium intake across countries, especially BMI and mode of administration. Above all, we suggest to additionally explore the between-center effect in the ranking of self-reported food groups and infrequently consumed nutrients across countries as well as the impact of using distinct modes of administration in the collection of dietary data across countries.
References
Riboli E, Hunt KJ, Slimani N, Ferrari P, Norat T, Fahey M, Charrondiere UR, Hemon B, Casagrande C, Vignat J, Overvad K, Tjonneland A, Clavel-Chapelon F, Thiebaut A, Wahrendorf J, Boeing H, Trichopoulos D, Trichopoulou A, Vineis P, Palli D, Bueno-De-Mesquita HB, Peeters PH, Lund E, Engeset D, Gonzalez CA, Barricarte A, Berglund G, Hallmans G, Day NE, Key TJ, Kaaks R, Saracci R (2002) European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr 5(6B):1113–1124
Pigeot I, Barba G, Chadjigeorgiou C, de Henauw S, Kourides Y, Lissner L, Marild S, Pohlabeln H, Russo P, Tornaritis M, Veidebaum T, Wawro N, Siani A (2009) Prevalence and determinants of childhood overweight and obesity in European countries: pooled analysis of the existing surveys within the IDEFICS Consortium. Int J Obes (Lond) 33(10):1103–1110
Moreno LA, Gonzalez-Gross M, Kersting M, Molnar D, de Henauw S, Beghin L, Sjostrom M, Hagstromer M, Manios Y, Gilbert CC, Ortega FB, Dallongeville J, Arcella D, Warnberg J, Hallberg M, Fredriksson H, Maes L, Widhalm K, Kafatos AG, Marcos A (2008) Assessing, understanding and modifying nutritional status, eating habits and physical activity in European adolescents: the HELENA (Healthy Lifestyle in Europe by Nutrition in Adolescence) Study. Public Health Nutr 11(3):288–299
EFSA (2009) General principles for the collection of national food consumption data in the view of a pan-European dietary survey. The EFSA Journal 7:1–51. http://www.efsa.europa.eu/en/efsajournal/doc/1435.pdf. Acessed 15 Jan 2011
Brussaard JH, Lowik MR, Steingrimsdottir L, Moller A, Kearney J, De Henauw S, Becker W (2002) A European food consumption survey method-conclusions and recommendations. Eur J Clin Nutr 56(Suppl 2):S89–S94
de Boer E (2011) Rationale and methods of the EFCOVAL project. Eur J Clin Nutr 65(Supp1):S1–S4
Kaaks RJ (1997) Biochemical markers as additional measurements in studies of the accuracy of dietary questionnaire measurements: conceptual issues. Am J Clin Nutr 65(4 Suppl):1232S–1239S
Ocke MC, Kaaks RJ (1997) Biochemical markers as additional measurements in dietary validity studies: application of the method of triads with examples from the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr 65(4 Suppl):1240S–1245S
Ferrari P, Roddam A, Fahey MT, Jenab M, Bamia C, Ocke M, Amiano P, Hjartaker A, Biessy C, Rinaldi S, Huybrechts I, Tjonneland A, Dethlefsen C, Niravong M, Clavel-Chapelon F, Linseisen J, Boeing H, Oikonomou E, Orfanos P, Palli D, Santucci de Magistris M, Bueno-de-Mesquita HB, Peeters PH, Parr CL, Braaten T, Dorronsoro M, Berenguer T, Gullberg B, Johansson I, Welch AA, Riboli E, Bingham S, Slimani N (2009) A bivariate measurement error model for nitrogen and potassium intakes to evaluate the performance of regression calibration in the European Prospective Investigation into Cancer and Nutrition study. Eur J Clin Nutr 63(Suppl 4):S179–S187
Tasevska N, Runswick SA, Bingham SA (2006) Urinary potassium is as reliable as urinary nitrogen for use as a recovery biomarker in dietary studies of free living individuals. J Nutr 136(5):1334–1340
Bingham SA, Cummings JH (1985) Urine nitrogen as an independent validatory measure of dietary intake: a study of nitrogen balance in individuals consuming their normal diet. Am J Clin Nutr 42(6):1276–1289
Slimani N, Bingham S, Runswick S, Ferrari P, Day NE, Welch AA, Key TJ, Miller AB, Boeing H, Sieri S, Veglia F, Palli D, Panico S, Tumino R, Bueno-De-Mesquita B, Ocke MC, Clavel-Chapelon F, Trichopoulou A, Van Staveren WA, Riboli E (2003) Group level validation of protein intakes estimated by 24-hour diet recall and dietary questionnaires against 24-hour urinary nitrogen in the European Prospective Investigation into Cancer and Nutrition (EPIC) calibration study. Cancer Epidemiol Biomarkers Prev 12(8):784–795
Crispim SP, de Vries JH, Geelen A, Souverein OW, Hulshof PJ, Lafay L, Rousseau AS, Lillegaard IT, Andersen LF, Huybrechts I, De Keyzer W, Ruprich J, Dofkova M, Ocke MC, de Boer E, Slimani N, Van’t Veer P (2011) Two non-consecutive 24 h recalls using EPIC-Soft software are sufficiently valid for comparing protein and potassium intake between five European centres—results from the European Food Consumption Validation (EFCOVAL) study. Br J Nutr 105(3):447–458
Hox J (2010) Multilevel analysis. Techniques and applications, 2nd edn. Routledge Academic, Utrecht
Slimani N, Kaaks R, Ferrari P, Casagrande C, Clavel-Chapelon F, Lotze G, Kroke A, Trichopoulos D, Trichopoulou A, Lauria C, Bellegotti M, Ocke MC, Peeters PH, Engeset D, Lund E, Agudo A, Larranaga N, Mattisson I, Andren C, Johansson I, Davey G, Welch AA, Overvad K, Tjonneland A, Van Staveren WA, Saracci R, Riboli E (2002) European Prospective Investigation into Cancer and Nutrition (EPIC) calibration study: rationale, design and population characteristics. Public Health Nutr 5(6B):1125–1145
Kaaks R, Plummer M, Riboli E, Esteve J, van Staveren W (1994) Adjustment for bias due to errors in exposure assessments in multicenter cohort studies on diet and cancer: a calibration approach. Am J Clin Nutr 59(1 Suppl):245S–250S
Ferrari P, Day NE, Boshuizen HC, Roddam A, Hoffmann K, Thiebaut A, Pera G, Overvad K, Lund E, Trichopoulou A, Tumino R, Gullberg B, Norat T, Slimani N, Kaaks R, Riboli E (2008) The evaluation of the diet/disease relation in the EPIC study: considerations for the calibration and the disease models. Int J Epidemiol 37(2):368–378
Slimani N, Deharveng G, Charrondiere RU, van Kappel AL, Ocke MC, Welch A, Lagiou A, van Liere M, Agudo A, Pala V, Brandstetter B, Andren C, Stripp C, van Staveren WA, Riboli E (1999) Structure of the standardized computerized 24-h diet recall interview used as reference method in the 22 centers participating in the EPIC project. European Prospective Investigation into Cancer and Nutrition. Comput Methods Programs Biomed 58(3):251–266
Slimani N, Ferrari P, Ocke M, Welch A, Boeing H, Liere M, Pala V, Amiano P, Lagiou A, Mattisson I, Stripp C, Engeset D, Charrondiere R, Buzzard M, Staveren W, Riboli E (2000) Standardization of the 24-hour diet recall calibration method used in the european prospective investigation into cancer and nutrition (EPIC): general concepts and preliminary results. Eur J Clin Nutr 54(12):900–917
Slimani N, Deharveng G, Unwin I, Southgate DA, Vignat J, Skeie G, Salvini S, Parpinel M, Moller A, Ireland J, Becker W, Farran A, Westenbrink S, Vasilopoulou E, Unwin J, Borgejordet A, Rohrmann S, Church S, Gnagnarella P, Casagrande C, van Bakel M, Niravong M, Boutron-Ruault MC, Stripp C, Tjonneland A, Trichopoulou A, Georga K, Nilsson S, Mattisson I, Ray J, Boeing H, Ocke M, Peeters PH, Jakszyn P, Amiano P, Engeset D, Lund E, de Magistris MS, Sacerdote C, Welch A, Bingham S, Subar AF, Riboli E (2007) The EPIC nutrient database project (ENDB): a first attempt to standardize nutrient databases across the 10 European countries participating in the EPIC study. Eur J Clin Nutr 61(9):1037–1056
FAO (2003) Food energy—methods of analysis and conversion factors, vol 77. Food and Agriculture Organization of the United Nations, Rome
Crispim SP, Geelen A, Le Donne C, De Vries JH, Sette S, Raffo A, Siebelink E, Ocke MC, van’t Veer P, Leclercq C (2010) Dietary exposure to flavouring substances: from screening methods to detailed assessments using food consumption data collected with EPIC-Soft software. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 27(4):433–446
Bingham SA, Williams R, Cole TJ, Price CP, Cummings JH (1988) Reference values for analytes of 24-h urine collections known to be complete. Ann Clin Biochem 25(Pt 6):610–619
Johansson G, Bingham S, Vahter M (1999) A method to compensate for incomplete 24-hour urine collections in nutritional epidemiology studies. Public Health Nutr 2(4):587–591
Bingham SA (2003) Urine nitrogen as a biomarker for the validation of dietary protein intake. J Nutr 133(Suppl 3):921S–924S
Holbrook JT, Patterson KY, Bodner JE, Douglas LW, Veillon C, Kelsay JL, Mertz W, Smith JC Jr (1984) Sodium and potassium intake and balance in adults consuming self-selected diets. Am J Clin Nutr 40(4):786–793
Haftenberger M, Lahmann PH, Panico S, Gonzalez CA, Seidell JC, Boeing H, Giurdanella MC, Krogh V, Bueno-de-Mesquita HB, Peeters PH, Skeie G, Hjartaker A, Rodriguez M, Quiros JR, Berglund G, Janlert U, Khaw KT, Spencer EA, Overvad K, Tjonneland A, Clavel-Chapelon F, Tehard B, Miller AB, Klipstein-Grobusch K, Benetou V, Kiriazi G, Riboli E, Slimani N (2002) Overweight, obesity and fat distribution in 50- to 64-year-old participants in the European Prospective Investigation into Cancer and Nutrition (EPIC). Public Health Nutr 5(6B):1147–1162
UNDP (2009) Human Development Report. Overcoming barriers: Human mobility and development. In: United Nations Development Programme. http://hdr.undp.org/en/. Accessed 30 Feb 2011
Kim S, Haines PS, Siega-Riz AM, Popkin BM (2003) The Diet Quality Index-International (DQI-I) provides an effective tool for cross-national comparison of diet quality as illustrated by China and the United States. J Nutr 133(11):3476–3484
Duncan C, Jones K, Moon G (1998) Context, composition and heterogeneity: using multilevel models in health research. Soc Sci Med 46(1):97–117
Diez Roux AV (2002) A glossary for multilevel analysis. J Epidemiol Community Health 56(8):588–594
EFSA (2010) Statistical modelling of usual intake. Sci report EFSA-Q-2009-00841:1–23
Rumpler WV, Kramer M, Rhodes DG, Moshfegh AJ, Paul DR (2008) Identifying sources of reporting error using measured food intake. Eur J Clin Nutr 62(4):544–552
Pryer JA, Vrijheid M, Nichols R, Kiggins M, Elliott P (1997) Who are the ‘low energy reporters’ in the dietary and nutritional survey of British adults? Int J Epidemiol 26(1):146–154
Subar AF, Kipnis V, Troiano RP, Midthune D, Schoeller DA, Bingham S, Sharbaugh CO, Trabulsi J, Runswick S, Ballard-Barbash R, Sunshine J, Schatzkin A (2003) Using intake biomarkers to evaluate the extent of dietary misreporting in a large sample of adults: the OPEN study. Am J Epidemiol 158(1):1–13
Heerstrass DW, Ocke MC, Bueno-de-Mesquita HB, Peeters PH, Seidell JC (1998) Underreporting of energy, protein and potassium intake in relation to body mass index. Int J Epidemiol 27(2):186–193
Lissner L, Troiano RP, Midthune D, Heitmann BL, Kipnis V, Subar AF, Potischman N (2007) OPEN about obesity: recovery biomarkers, dietary reporting errors and BMI. Int J Obes (Lond) 31(6):956–961
Halkjaer J, Olsen A, Bjerregaard LJ, Deharveng G, Tjonneland A, Welch AA, Crowe FL, Wirfalt E, Hellstrom V, Niravong M, Touvier M, Linseisen J, Steffen A, Ocke MC, Peeters PH, Chirlaque MD, Larranaga N, Ferrari P, Contiero P, Frasca G, Engeset D, Lund E, Misirli G, Kosti M, Riboli E, Slimani N, Bingham S (2009) Intake of total, animal and plant proteins, and their food sources in 10 countries in the European Prospective Investigation into Cancer and Nutrition. Eur J Clin Nutr 63(Suppl 4):S16–S36
Casey PH, Goolsby SL, Lensing SY, Perloff BP, Bogle ML (1999) The use of telephone interview methodology to obtain 24-hour dietary recalls. J Am Diet Assoc 99(11):1406–1411
Fox TA, Heimendinger J, Block G (1992) Telephone surveys as a method for obtaining dietary information: a review. J Am Diet Assoc 92(6):729–732
Brustad M, Skeie G, Braaten T, Slimani N, Lund E (2003) Comparison of telephone vs face-to-face interviews in the assessment of dietary intake by the 24 h recall EPIC SOFT program-the Norwegian calibration study. Eur J Clin Nutr 57(1):107–113
Crispim SP, Geelen A, de Vries JH, Souverein OW, Hulshof PJ, Lafay L, Rousseau AS, Lillegaard IT, Andersen LF, Huybrechts I, De Keyzer W, Ruprich J, Dofkova M, Ocke MC, de Boer E, Slimani N, Van’t Veer P (2011) Biomarker-based evaluation of two 24-h recalls (EPIC-Soft) for estimating and comparing fish, fruit and vegetable intakes across five European centers: results from the European Food Consumption Validation (EFCOVAL) study. Eur J Clin Nutr 65(Suppl 1):S38–S47
Acknowledgments
We wish to thank Aurelie Moskal, Beatrice Vozar, Bertrand Hemon, Carine Biessy, Graham Byrnes, Jérôme Vignat, Pietro Ferrari, Sabina Rinaldi, Stephen Sharp, Veronique Chajes and Thomas Cler for their helpful comments and discussions. SC carried out data analyses and wrote the paper, taking into account comments from all coauthors. NS and PV had primary responsibility for the final content. JV, AG, HF, OS, PH, MO, HB, LA, JR, WK, IH, LL, MM, FR RT, VK, HBM, JB, MR, AN, FC, HB, AM and RK were local EPIC and EFCOVAL collaborators involved in the data collection and gave input on interpretation of results. All coauthors commented on the paper and approved the final version. The work reported in this paper was undertaken while SC was hosted by the International Agency for Research on Cancer (IARC). The EFCOVAL Study took place with the financial support of the European Community Sixth RTD Framework Programme (Contract FOOD-CT-2006-022895). The content of the paper reflects only the authors’ views, and the Community is not liable for any use that may be made of the information contained therein. Ruprich received consulting fees from the Czech Technology Platform for food and health lifestyle and Naska financial support from the Hellenic Ministry of Health and the Hellenic Health Foundation.
Conflict of interest
Crispim, Geelen, de Vries, Freisling, Souverein, Hulshof, Ocke, Boshuizen, Andersen, De Keizer, Huybrechts, Lafay, Magistris, Ricceri, Tumino, Krogh, Bueno-de-Mesquita, Beulens, Boutron-Ruault, Crowe, Boeing, McTaggart, Kaaks, van’t Veer and Slimani have declared no conflicts of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix: Specification of models used in the multilevel approach
Appendix: Specification of models used in the multilevel approach
The following regression model represents model iii (random intercepts with individual- and center-level explanatory variables) in the assessment:
where, j = the index for the centers (j = 1,…, N), i = the index for the individuals within the centers (i = 1,…, n j ), Y ij : log ratio between dietary intake and biomarker for ith individual in the jth center, α: the overall mean of log ratio between intake and biomarker across all centers, β1,…, β n : effects of individual explanatory variables X 1ij ,…, X nij , γ1,…, γ n : fixed effects of the center-level explanatory variables Z 1j ,…, Z nj , u 0j : center-level random effects on the mean of the intercept of Y, e ij : residual error term, assumed to have a mean of zero and a variance (\( \sigma_{e}^{2} \) = individual random effect), Thus, this model has fixed-effect parameters (α, β n , γ n ) as well as zero-mean random coefficients (u 0j , e ij ).
In model ii (random intercepts with only individual explanatory variables), the coefficients γ1,…, γ n of the center-level variables Z 1j ,…, Z nj are zero. Model i additionally constrained to zero the coefficients β1,…, βn from individual variables X 1ij ,…, X nij .
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Crispim, S.P., Geelen, A., de Vries, J.H.M. et al. Bias in protein and potassium intake collected with 24-h recalls (EPIC-Soft) is rather comparable across European populations. Eur J Nutr 51, 997–1010 (2012). https://doi.org/10.1007/s00394-011-0279-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-011-0279-z