Abstract
The parasitoid wasp Apanteles taragamae is a promising candidate for the biological control of the legume pod borer Maruca vitrata, which recently has been introduced into Benin. The effects of volatiles from cowpea and peabush flowers and Maruca vitrata larvae on host selection behavior of the parasitoid Apanteles taragamae were investigated under laboratory conditions by using a Y-tube olfactometer. Naïve and oviposition-experienced female wasps were given a choice between several odor sources that included (1) uninfested, (2) Maruca vitrata-infested, and (3) mechanically damaged cowpea flowers, as well as (4) stem portions of peabush plants carrying leaves and flowers, (5) healthy M. vitrata larvae, and moribund (6), and live (7) virus-infected M. vitrata larvae. Responses of naïve and oviposition-experienced female wasps did not differ for any of the odor source combinations. Wasps were significantly attracted to floral volatiles produced by cowpea flowers that had been infested with M. vitrata larvae and from which the larvae had been removed. Apanteles taragamae females also were attracted to Maruca vitrata-infested flowers after removal of both the larvae and their feces. Female wasps discriminated between volatiles from previously infested flowers and mechanically damaged flowers. Uninfested cowpea flowers attracted only oviposition-experienced wasps that had received a rewarding experience (i.e. the parasitization of two M. vitrata larvae feeding on cowpea flowers) before the olfactometer test. Wasps also were attracted to uninfested leaves and flowers of peabush. Moreover, they were also attracted to healthy and live virus-infected M. vitrata larvae, but not when the latter were moribund. Our data show that, similarly to what has been extensively been reported for foliar volatiles, flowers of plants also emit parasitoid-attracting volatiles in response to being infested with an herbivore.
Similar content being viewed by others
Introduction
The impact of insect parasitoids on host populations depends on abiotic and biotic factors. Host searching capacity is considered to be an important component of parasitoid biology, often influencing the success of inoculative biological control (Gilstrap, 1997; Wiedenmann and Smith, 1997; Van Lenteren and Manzaroli, 1999; Neuenschwander, 2001; Vet, 2001; Gross et al., 2005). Effective location of hosts is especially important at low host densities. Foraging parasitoids have to deal with the so-called detectability-reliability problem (Vet and Dicke, 1992). Stimuli provided by herbivorous insects (hosts) are reliable indicators of their presence to parasitoids, but the detectability of these cues is low. On the other hand, stimuli emitted by the food plants of their herbivorous hosts are more detectable because they are emitted in larger amounts, but they do not necessarily indicate the presence of the herbivores (Vet and Dicke, 1992). In order to cope with this problem, parasitoids may combine stimuli from both their hosts and host food plants (Turlings et al., 1991a, 1993; Vet and Dicke, 1992; Dicke, 1999a, b; Fatouros et al., 2005; Tamò et al., 2006). Natural enemies, such as insect parasitoids, use infochemicals from host plants to locate the habitat of their hosts (Vinson, 1976; Dicke and Sabelis, 1988; Turlings et al., 1990; Vet and Dicke, 1992; Dicke, 1999b; Mumm and Hilker, 2006; Schnee et al., 2006; Heil, 2008). Natural enemies are able to discriminate between blends of volatiles produced by mechanically damaged and herbivore-damaged plants (Eller et al., 1988; Turlings et al., 1991b; Vet and Dicke, 1992; Dicke, 1999a). In addition to herbivore-induced plant volatiles, several other factors also may affect host searching behavior of a parasitoid, such as visual and vibrational stimuli from the host or host plant, the presence of competitors or natural enemies, previous oviposition experiences (learning), and physiological state of the parasitoid (Lawrence, 1981; Wardle, 1990; Wardle and Borden, 1990; Wäckers and Lewis, 1994; Casas et al., 1998; Dicke, 1999b; Dicke and Grostal, 2001).
The success of biological control agents in many projects has been attributed in particular to high host searching efficiency (Neuenschwander and Ajuonu, 1995; Ngi-Song and Overholt, 1997; de Moraes et al., 1999; Neuenschwander, 2001). For instance, the superiority of Apoanagyrus (Epidinocarsis) lopezi De Santis over Apoanagyrus diversicornis Howard (which is more fecund than A. lopezi) was attributed to its higher capacity to locate and parasitize young host instars at low densities (Neuenschwander, 2001). Similarly, Cardiochiles nigriceps Viereck displayed a higher host searching capacity, and consequently detected and parasitized more larvae of Heliothis virescens (Fabricius) compared to Microplitis croceipes Cresson (de Moraes et al., 1999).
Apanteles taragamae Viereck, the parasitoid that was used in the present study, is a solitary larval endoparasitoid of the legume pod borer Maruca vitrata Fabricius (Lepidoptera: Crambidae). It parasitized on average 63% of M. vitrata larvae on Sesbania cannabina (Retz) Pers. (Huang et al., 2003). The wasp also can transmit the multi-nucleopolyhedrovirus MaviMNPV to larvae of M. vitrata (M. Tamò, personal communication).
Maruca vitrata is one of the key insect pests of cowpea, causing up to 80% of yield loss (Nampala et al., 2002). Damage by M. vitrata to grain legumes is made by its larvae (Taylor, 1978). Larvae feed on flower buds, flowers, and pods of cowpea (Taylor, 1978; Sharma, 1998). Infestation of flowers was found to be higher than that of flower buds and pods (Sharma, 1998). Larvae of this crambid also were reported to damage leaves of some wild leguminous plants, such as the peabush, S. cannabina (Huang et al., 2003).
An attempt to exert biological control of this insect pest is made through the importation of A. taragamae from Taiwan into Benin by the International Institute of Tropical Agriculture (IITA). The potential of the wasp as biological control agent is being evaluated.
In this study, we assessed the role of volatiles produced by M. vitrata larvae and two host plants, cowpea and peabush, in the host selection process by the parasitoid wasp A. taragamae by using a Y-tube olfactometer. Cowpea is the main cultivated host plant of M. vitrata in Benin whereas peabush is the host plant on which the parasitoid was collected in Taiwan.
Methods and Materials
Plant materials
Seeds of the local cowpea variety Kpodji-guêguê and of peabush were sown in potted soil. Pots were placed in a greenhouse at 28 ± 1°C, and 76 ± 6% relative humidity (means ± SD). Plants were watered every 3 days during the experimental period. Experiments started at the onset of flowering. Flowers or stem portions were collected from cowpea (Vigna unguiculata) or peabush (Sesbania cannabina) plants to prepare the different odor sources used in the olfactometer tests.
Insect Materials
Mass rearing of Maruca vitrata
Pupae of M. vitrata were obtained from a stock culture at the field station of the International Institute of Tropical Agriculture (IITA) in Benin. They were placed in open Petri dishes that were incubated in wooden cages (44 × 45 × 58 cm) with sleeves, having sides of fine screen and a glass top, and kept at 27.0 ± 0.6°C and 60.9 ± 4.6% relative humidity (means ± SD). Adults emerged inside the cages and were fed by using cotton fibers moistened with 10% glucose solution. Four-d-old female moths were transferred in groups of four or five individuals to transparent cylindrical plastic cups (3 cm diam. × 3.5 cm high) and kept for 24 h to allow for oviposition, which occurred on the inner surface of the cups. Ovipositing females were fed with small pieces of filter paper moistened with 10% glucose solution, which were replaced every 24 h. Cups carrying eggs were kept under the same experimental conditions until the larvae hatched. Larvae were transferred to large cylindrical plastic containers (9 cm diam. × 12 cm high) provided with artificial diet prepared according to Jackai and Raulston (1988), and developed through five instars until pupation. The artificial diet contained 4 l water; 59.2 g Agar-agar; 400 g cowpea grain flour; 127.2 g wheat or maize germ flour; 60 g Wesson salt; 44.4 g sorbic acid; 6.3 g methyl p-hydroxy-benzoate; 25 g ascorbic acid; 50 ml acetic acid; 6 ml formaldehyde; 11 g aureomicin; 22 g potassium hydroxide; 29.6 ml choline chloride; and 30 ml vitamin B mixture (Jackai and Raulston, 1988). Pupae were collected and placed in cages. All larvae used in the experiments were obtained from the mass production.
Mass rearing of Apanteles taragamae
Cocoons of A. taragamae were obtained from the stock culture at the IITA station in Benin that originated from parasitoids collected on S. cannabina infested by M. vitrata at the World Vegetable Center (AVRDC) in Taiwan. Emerged adults were kept in plastic cylindrical cups (4.5 cm diam. × 5 cm high). A hole (2 cm diam.) punched in the lid of the cups was covered with fine mesh. Adults of A. taragamae were fed with honey streaked on the fine mesh of the lid. To allow mated female wasps to parasitize hosts, they were offered, during 24 h, 2-d-old larvae of M. vitrata in a small cylindrical cup (3 cm diam. × 3.5 cm high) containing a piece of artificial diet. The exposed larvae were reared until cocoon stage. Cocoons were collected and placed in cylindrical cups (4.5 cm diam. × 5 cm high). Mass production of wasps took place in a climate chamber with a temperature of 25.3 ± 0.5°C (mean ± SD) and a relative humidity of 78.9 ± 5.6% (mean ± SD). Female wasps used for the different choice tests were obtained from this mass rearing.
Maruca vitrata Multi-Nucleopolyhedrovirus (MaviMNPV)
Maruca vitrata multi-nucleopolyhedrovirus (MaviMNPV) is a baculovirus isolated from infected larvae of M. vitrata on peabush in Taiwan (Lee et al., 2007; Chen et al., 2008). Infected larvae were sluggish, pinkish, and ceased feeding 3–4 day after virus exposure. When dead, larvae were found hanging from the top of the plant with the prolegs attached to the host plant. The virus attacks all larval stages with a high susceptibility of early instars (first and second stages). MaviMNPV potentially could be used as a component in an Integrated Pest Management Programme against M. vitrata (Lee et al., 2007). It has been introduced to the IITA-Benin laboratory from AVRDC for experimental purposes.
Oviposition-experienced female wasps
Emerged adult female parasitoids were kept together with males for 48 h in cylindrical plastic cups (4.5 cm diam. × 5 cm high) to allow mating. They were fed with honey. Mated females gained oviposition experience by parasitizing two 2-d-old M. vitrata larvae in cylindrical plastic cups (3 cm diam. × 3.5 cm high), 30 min prior to the olfactometer tests. The host larvae had been reared on artificial diet. These oviposition-experienced parasitoid females had not received contact with the odor sources used in the present study.
Odor-experienced female wasps
Two-d-old mated female parasitoids were kept together with uninfested cowpea flowers for 24 h in cylindrical plastic containers (9 cm diam × 12 cm high) where they were fed with honey streaked on the mesh cover of containers. Thirty min prior to the olfactometer test, they were allowed to parasitize 2-d-old larvae feeding on cowpea flowers. Odor-experienced females were used only to test for their response to the volatiles from uninfested cowpea flowers against clean air.
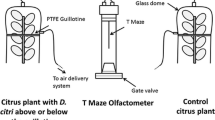
Dynamic olfactometer set-up
The response of A. taragamae females to volatiles produced by cowpea, peabush flowers, and host larvae was investigated by using a glass Y-tube olfactometer similar to that used by Gnanvossou et al. (2003). Clean airflow was divided in two, and each subflow passed through one of the two odor sources connected to the arms of the glass Y-tube olfactometer. The windspeed in the olfactometer was controlled at 4 l/min.
Bioassay procedure
Naïve mated females of A. taragamae (without oviposition experience) and oviposition-experienced wasps alternatingly were introduced individually at the entry of the Y-shaped glass tube. Their movement was observed for maximally 10 min. A test began when the wasp started to move. Female wasps remaining motionless for more than 5 min at the release point were discarded from the analysis. Parasitoid wasps that did not reach the end of the olfactometer arm were considered as non-responding wasps. After testing two naïve and two oviposition-experienced female parasitoids, the positions of the odor sources were exchanged to correct for any unforeseen asymmetry in the experimental set-up. Odor sources were renewed after testing eight naïve and eight experienced female wasps. A total of 16–20 naïve and oviposition-experienced female wasps were tested daily, and 60–70 naïve and oviposition-experienced females were tested in total for each choice situation. All female wasps used in this study were 3-d-old.
Bioassays on the response of naïve and oviposition-experienced female wasps to volatiles produced by cowpea flowers
The influence of volatiles produced by cowpea flowers on the host selection behavior of both naïve and oviposition-experienced females of A. taragamae was investigated by testing the following odor combinations: a) 4 uninfested flowers vs. clean air; b) 4 caterpillar-infested flowers from which larvae were removed prior to the experiment vs. clean air; c) 4 caterpillar-infested flowers from which larvae and their feces were removed prior to the experiment vs. clean air; d) 4 mechanically damaged flowers vs. clean air; e) 4 uninfested vs. 4 caterpillar-infested flowers from which larvae were removed prior to the experiment; f) 4 uninfested vs. 4 mechanically damaged flowers; g) 4 caterpillar-infested flowers from which larvae and feces were removed prior to the experiment vs. 4 uninfested flowers; h) 4 caterpillar-infested flowers from which larvae were removed prior to the experiment vs. 4 mechanically damaged flowers.
Infested cowpea flowers consisted of racemes carrying 4 flowers, infested with 10 1-d-old larvae of M. vitrata for 24 h. Before the infestation, racemes were placed in water-filled cylindrical plastic vials (4.5 cm diam × 11.5 cm high) sealed with parafilm to keep racemes fresh and hydrated. Larvae were removed 15 min prior to using the flowers in an olfactometer experiment. Flowers were mechanically damaged 15 min prior to the experiment by three scratched lines onto flowers by using a clean needle.
Bioassays assessing the influence of previous contact with cowpea and peabush plants on the host searching behavior of A. taragamae
The influence of an odor experience on the behavioral response to uninfested flowers was studied by using odor-experienced female wasps for odor combination (a) (clean air vs. uninfested cowpea flowers).
As the wasp strain in use was collected originally on peabush plants, the effect of leaves and flowers of this plant on the host searching behavior of the wasp was evaluated by using both naïve and oviposition-experienced females. The M. vitrata larvae cause damage to peabush mostly by destroying leaves (Huang et al., 2003). However, flowers of this leguminous shrub also may be damaged. Both naïve and oviposition-experienced female wasps were used to test the following odor combination: i) Uninfested stem portions carrying 4 leaves and 4 flowers of S. cannabina vs. clean air.
Bioassays on the effect of volatiles from host larvae on the host searching behavior of A. taragamae
The effect of volatiles produced by M. vitrata larvae on the host selection behavior of A. taragamae was assessed by using healthy, moribund, and live MaviMNPV-infected larvae. Virus-infection had occurred during the mass rearing of A. taragamae. Our objective was to assess whether the wasps, being capable of transmitting the baculovirus, avoid infected larvae. Both naïve and oviposition-experienced female wasps were used to test the following odor combinations: j) 10 healthy larvae vs. clean air; k) 10 moribund MaviMNPV-infected larvae vs. clean air; l) 10 healthy larvae vs. 10 moribund MaviMNPV-infected larvae; m) 10 MaviMNPV-infected larvae vs. clean air; n) 10 MaviMNPV-infected larvae vs. 10 healthy larvae.
Live MaviMNPV-infected larvae were obtained by feeding larvae with virus-infected artificial diet for 2 days. For this, pieces of artificial diet were placed in a viral suspension of 2 × 104 OB/ml (Occluded Bodies). Moribund larvae were larvae that naturally occurred in the mass rearing of M. vitrata and that had viral infection symptoms. The numbers of parasitoids that chose each odor source as first and final choice were recorded.
Statistical analysis
Analysis of data on the number of parasitoids per odor source was performed by using binomial tests with the null hypothesis that the distribution of the wasps over the two arms of the olfactometer was 50:50. Differences between naïve and experienced females wasps were tested with a 2 × 2 contingency table analysis based on Chi-square. Non-responding wasps were recorded but not included in the statistical analysis.
Results
Influence of volatiles from cowpea flowers on the host searching behavior of A. taragamae
In all experiments, the responses of the naïve and the oviposition-experienced wasps were not significantly different (contingency table tests, P > 0.05). Therefore, we combined the data for naïve and oviposition-experienced wasps.
Females of A. taragamae were significantly attracted to volatiles from M. vitrata-infested cowpea flowers from which larvae had been removed prior to the experiment, when tested against clean air or uninfested flowers (Fig. 1). Similar results were obtained when the wasps were offered infested flowers from which larvae and feces had been removed prior to the experiment, against clean air or uninfested flowers. The parasitoids did not discriminate between volatiles from uninfested flowers and clean air, between volatiles from mechanically damaged flowers and clean air, or between volatiles from mechanically damaged and uninfested flowers (Fig. 1). Female wasps did discriminate between volatiles from M. vitrata-infested flowers and mechanically damaged flowers (Fig. 1).
Behavioral response of Apanteles taragamae females offered choices between volatiles from cowpea flowers, that were either uninfested, mechanically damaged, or infested with Maruca vitrata, and clean air in a Y-tube olfactometer. Numbers in bars represent the total number of parasitoids that chose the olfactometer arm. P-values given to the right of the bars are for the two-tailed binomial test
Response to volatiles produced by uninfested peabush and cowpea flowers
Volatiles from stem portions carrying uninfested leaves and flowers of the peabush S. cannabina attracted A. taragamae females when tested against clean air (Fig. 2). When wasps were given an odor experience, uninfested cowpea flowers were preferred over clean air (Fig. 3), while without the odor experience there was no effect of volatiles from uninfested flowers on parasitoid attraction (Fig. 1).
Behavioral response of odor-experienced females of Apanteles taragamae to uninfested cowpea flowers in a Y-tube olfactometer. Numbers in bars represent the total number of parasitoids that chose olfactometer arm. P-value given to the right of bars is for the two-tailed binomial test. *: Odor-experienced female wasps are females that that had parasitized two 2-d-old larvae reared in the presence of host-infested cowpea flowers, 30 min prior to the olfactometer test
Response to volatiles from host larvae
Females of A. taragamae displayed a preference for volatiles produced by healthy M. vitrata larvae when tested against clean air (Fig. 4). Wasps did not show any preference for volatiles from moribund MaviMNPV-infected larvae over clean air, and they preferred volatiles emitted by healthy larvae over volatiles from moribund MaviMNPV-infected larvae. The parasitoid females did discriminate volatiles from live MaviMNPV-infected larvae against clean air (Fig. 4), but no preference was displayed when they were given a choice between volatiles from healthy larvae and live MaviMNPV-infected larvae.
Behavioral response of Apanteles taragamae females offered choices between volatiles from healthy Maruca vitrata larvae, moribund or live MaviMNPV-infected larvae, and clean air in a Y-tube olfactometer. Numbers in bars represent the total number of parasitoids that chose olfactometer arm. P-values given to the right of bars are for the two-tailed binomial test
Discussion
Results from this study show the importance of M. vitrata-induced floral volatiles produced by cowpea in the host selection process of A. taragamae. Female wasps were attracted by volatiles emitted by M. vitrata-infested cowpea flowers from which the larvae had been removed. The parasitoids were not attracted to uninfested cowpea flowers, but this changed when they had received an odor experience. Indeed, long-range volatiles produced by undamaged or herbivore-damaged plants are known to attract natural enemies of herbivorous insects, thus increasing their efficiency in locating their hosts’ habitat (Dicke and Sabelis, 1988; Turlings et al., 1990; Vet and Dicke, 1992; Ngi-Song et al., 1996; Du et al., 1998; Dicke, 1999b; Shimoda et al. 2005; Moayeri et al., 2007; Dicke and Baldwin, 2010). In most cases, studies have addressed the volatiles that are produced by leaves. In our study, however, volatiles were emitted by the previously infested flowers. Floral volatiles are known primarily as attractants for pollinators (Jervis et al., 1993; Pichersky and Gershenzon, 2002). However, herbivorous insects that feed or oviposit on flowers have been reported to rely on cues from flowers to locate their hosts (Ekesi et al., 1998; Jönsson et al., 2005; Andrews et al., 2007), and parasitoids may also use floral volatiles to locate a food source (nectar) (Wäckers, 2004). However, to our knowledge only one other study has shown that herbivore-damaged flowers emit volatiles that attract a parasitoid enemy of florivorous herbivores (Jönsson and Anderson, 2008). It will be interesting to investigate how volatiles from herbivore-infested flowers affect the behavior of pollinators. After all, herbivore-infested flowers are likely to be an inferior food source to pollinators.
The attraction of A. taragamae to uninfested peabush leaves and flowers may be a reflection of a genetic trait related to searching in peabush fields. Indeed, the current wasp species was imported from Taiwan, where it was collected from M. vitrata larvae feeding on peabush leaves. Volatiles from uninfested plants have been reported to be long-range attractants in some other parasitoid species (Elzen et al., 1983; Ding et al., 1989; Ngi-Song et al., 1996). Thus, Cotesia flavipes Cameron, originally collected in maize showed a preference for uninfested maize plants over sorghum in a dual choice olfactometer experiment (Ngi-Song et al., 1996). Likewise, the endogenous parasitoid C. sesamiae (Cameron) preferred volatiles produced by uninfested sorghum plants over those from maize. Indeed, both sorghum and C. sesamiae originate from Africa and share the same environment. The braconid Macrocentrus grandii Goidanish, a larval parasitoid of the European corn borer, Ostrinia nubilalis (Hubner), was attracted to uninfested maize plants (Ding et al., 1989). However, herbivore-infested plants were found to be more attractive than uninfested plants (Turlings et al., 1991a; Du et al., 1998; Vet et al., 1998; Dicke, 1999b).
In the host habitat, short-range stimuli from the host itself are reliable indicators of its presence, but they usually are not well detectable (Vet and Dicke, 1992). Our study shows that the parasitoid wasp A. taragamae was significantly attracted to volatiles emitted by M. vitrata larvae. An oviposition experience through the parasitization of larvae fed with artificial diet did not affect the parasitoid’s response to host larval volatiles. The use of kairomones by parasitoids for host location has been reported in many parasitoid species (Afsheen et al., 2008). The braconid parasitoid Microplitis. croceipes was attracted to odors of Heliothis virescens (Fabricius) larvae (Elzen et al., 1987; Röse et al., 1997). Similarly, the bruchid larval parasitoid Eupelmus vuilleti (Crw) was reported to respond to volatiles from Bruchidius atrolineatus (Pic) larvae (Cortesero and Monge, 1994). However, usually herbivore-induced plant volatiles are more attractive to parasitoids than herbivore-produced volatiles (Turlings et al., 1991a; Steinberg et al., 1992).
Herbivore-associated organisms such as microbes also may be a source of chemical information to parasitoids during host location (Vet and Dicke, 1992). Apanteles taragamae has been found to be a vector for the transmission of MaviMNPV to larvae of M. vitrata and could acquire and transmit the virus over several generations (M. Tamò, personal communication). In this study, females of A. taragamae were attracted to MaviMNPV-infected live larvae, but not to moribund larvae (Fig. 4). The wasps did not discriminate between volatiles from healthy and MaviMNPV-infected live larvae, but they preferred the volatiles from healthy larvae over those from moribund larvae. Apparently, the viral infection only affected larvae attractiveness in a late stage of infection. The virus disease symptoms appear about 3–4 day after infection of the M. vitrata larvae (Lee et al., 2007). Our observations are similar to those reported for the parasitoid Biosteres longicaudatus Ashmead, which was unable to locate immobilized or dead hosts (Lawrence, 1981).
MaviMNPV, like other baculoviruses, which are host-destroying viruses, is likely to affect negatively the development of A. taragamae, and it needs more attention for its management. Parasitoids and insect pathogens often are involved in scramble competition for host resources in dually infected and parasitized hosts. In such cases, some parasitoid species develop a strategy to enhance their developmental rate (Escribano et al., 2000). The temperature seemed to influence MaviMNPV symptoms development in parasitized larvae. At 29°C, A. taragamae pupated on average 6 d after parasitization, and this limited the detrimental effect of the virus infection (Dannon et al. unpublished data). Interactions between the wasp and MaviMNPV need to be investigated further to evaluate the effects of the virus on the parasitoid and to identify factors that avoid or limit the detrimental effect on the wasp reproduction.
In summary, this study shows that floral volatiles produced by M. vitrata-infested cowpeas flowers attracted A. taragamae females. Uninfested leaves and flowers of peabush also attracted the parasitoid. In contrast, the wasp was attracted to uninfested cowpea flower only after an odor experience. Olfactory cues from M. vitrata larvae also were used by the wasp in its host selection process. Further research should assess the influence of other key host plants of M. vitrata, such as Pterocarpus santalinoides L’herit ex DC., and Lonchocarpus sericeus (Poir) H.B.K. on the host selection behavior of A. taragamae.
References
Afsheen, S., Wang, X., Li, R., Zhu, C-S., and Lou, Y-G. 2008. Differential attraction of parasitoids in relation to specificity of kairomones from herbivores and their by-products. Insect Sci. 15:381–397.
Andrews, E. S., Theis, N., and Adler, L. S. 2007. Pollinator and herbivore attraction to Cucurbita floral volatiles. J. Chem. Ecol. 33:1682–1691.
Casas, J., Bacher, S., Tautz, J., Meyhofer, R., and Pierre, D. 1998. Leaf vibrations and air movements in a leafminer-parasitoid system. Biol. Control 11:147–153.
Chen, Y.-C., Wu, C.-Y., Lee, S.-T., Wu, Y.-J., Lo, C.-F., Tsai, M.-F., and Wang, C.-H. 2008. Genomic and host range studies of Maruca vitrata nucleopolyhedrovirus. J. Gen. Virol. 89:2315–2330.
Cortesero, A. M., and Monge, J. P. 1994. Influence of pre-emergence experience on response to host and host plant odors in the larval parasitoid Eupelmus vuilleti. Entomol. Exp. Appl. 72:281–288.
De Moraes, C., Cortesero, A. M., Stapel, J. O., and Lewis, W. J. 1999. Intrinsic and extrinsic competitive interactions between two larval parasitoids of Heliothis virescens. Ecol. Entomol. 24:402–410.
Dicke, M. 1999a. Direct and indirect effects of plants on the performance of beneficial organisms, pp. 105–153, in J. R. Ruberson (ed.). Handbook of Pest Management. Marcel Dekker, Inc, New York.
Dicke, M. 1999b. Are herbivore-induced plants volatiles reliable indicators to herbivores identity to foraging carnivorous arthropods? Entomol. Exp. Appl. 91:131–142.
Dicke, M., and Baldwin, I. T. 2010. The evolutionary context for herbivore-induced plant volatiles: beyond the ‘cry-for-help’. Trends Plant Sci. 15:167–175.
Dicke, M. and Grostal, P. 2001. Chemical detection of natural enemies by arthropods: an ecological perspective. Annu. Rev. Ecol. Syst. 32:1–23.
Dicke, M., and SABELIS, M. W. 1988. Infochemical terminology: based on cost-benefit analysis rather than origin of compounds. Funct. Ecol. 2:131–139.
Ding, D., Swedenborg, P. D., and Jones, R. L. 1989. Chemical stimuli in host-seeking behaviour of Macrocentus grandii (Hymenoptera: Ichneumonidae). Ann. Entomol. Soc. Am. 82:232–236.
Du, Y., Poppy, G. M., Powell, W., Pickett, J. A., Wadhams, L. J., and Woodcock, C. M. 1998. Identification of semiochemicals released during aphid feeding that attract parasitoid Aphidius ervi. J. Chem. Ecol. 24:1355–1368.
Ekesi, S., Maniania, N. K., and Onu, I. 1998. Antibiosis and antixenosis of two cowpea varieties of the legume flower thrips. Afric. Crop Sci. J. 6:49–59.
Eller, F. J., Tumlinson, J. H., and Lewis, W. J. 1988. Beneficial arthropod behavior mediated by airborne semiochemicals II. Olfactometric studies of host location by the parasitoid Microplitis croceipes (Cresson) (Hymenoptera: Braconidae). J. Chem. Ecol. 14:425–434.
Elzen, G. W., Williams, H. J., and Vinson, S. B. 1983. Response by the parasitoid Campoletis sonorensis (Hymenoptera: Ichneumonidae) to chemicals (synomones) in plants: Implications for host habitat location. Environ. Entomol. 12:1873–1877.
Elzen, G. W., Williams, H. J., Vinson, S. B., and Powell, J. E. 1987. Comparative flight behavior of parasitoid Campoletis sonorensis and Microplitis croceipes. Entomol. Exp. Appl. 45:175–180.
Escribano, A., Williams, T., Goulson, D., Cave, R. D., and Caballero, P. 2000. Parasitoid-pathogen-pest interactions of Chelonus insularis, Campoletis sonorensis, and a nucleopolyhedrovirus in Spodoptera frugiperda larvae. Biol. Control 19:265–273.
Fatouros, N. E., Van Loon, J. J. A., Hordijk, K. A., Smid, H. M., and Dicke, M. 2005. Herbivore-induced plant volatiles mediate in flight host discrimination by parasitoids. J. Chem. Ecol. 31:2033–2047.
Gilstrap, F. E. 1997. Importation biological control in ephemeral crop habitats. Biol. Control 10:23–29.
Gnanvossou, D., Hanna, J. R., and Dicke, M. 2003. Infochemical-mediated niche use by the predatory mites Typhlodromalus manihoti and T. aripo (Acari: Phytoseiidae). J. Insect Behav. 16:523–535.
Gross, P., Hawkins, B. A., Cornell, H. V., and Hosmane, B. 2005. Using lower trophic level factors to predict outcomes in classical biological control of insect pests. Basic Appl. Ecol. 6:571–584.
Heil, M. 2008. Indirect defence via tritrophic interactions. New Phytol. 178:41–61.
Huang, C.-C., Peng, W.-K., and Talekar, N. S. 2003. Parasitoids and other natural enemies of Maruca vitrata feeding on Sesbania cannabina in Taiwan. BioCont. 48:407–416.
Jackai, L. E. N., and Raulston, J. R. 1988. Rearing the legume pod borer, Maruca testulalis Geyer (Lepidoptera: Pyralidae) on artificial diet. Trop. Pest Manag. 34:168–172.
Jervis, M. A., Kidd, N. A. C., Fitton, M. G., Huddleston, T., and Dawah, H. A. 1993. Flower visiting by hymenopteran parasitoids. J. Nat. Hist. 27:67–105.
Jönsson, M., and Anderson, P. 2008. Emission of oilseed rape volatiles after pollen beetle infestation; behavioural and electrophysiological responses in the parasitoid Phradis morionellus. Chemoecology 17:201–207.
Jönsson, M., Lindkvist, A., and Anderson, P. 2005. Behavioural responses in three ichneumonid pollen beetle parasitoids to volatiles emitted from different phenological stages of oilseed rape. Entomol. Exp. Appl. 115:363–369.
Lawrence, P. O. 1981. Host vibration—A cue to host location by the parasite Biosteres longicaudatus. Oecologia 48:249–251.
Lee, S.-T., Srinivasan, R., Wu, Y.-J., and Talekar, N. S. 2007. Occurrence and characterization of a nucleoplyhedrovirus from Maruca vitrata (Lepidoptera: Pyralidae) in Taiwan. BioCont. 52:801–819.
Moayeri, H. R. S., Ashouri, A., Poll, L., and Enkegaard, A. 2007. Olfactory response of a predatory mirid to herbivore induced plant volatiles: multiple herbivory vs. single herbivory. J. Appl. Entomol. 131:326–332.
Mumm, R., and Hilker, M. 2006. Direct and indirect chemical defence of pine against folivorous insects. Trends Plant Sci. 11:351–358.
Nampala, P., Ogenga-Latigo, M. W., Kyamanywa, S., Adipala, E., Oyodo, M., and Jackai, L. E. N. 2002. Potential impact of intercropping on major cowpea field pests in Uganda. Afr. Crop Sci. J. 10:335–344.
Neuenschwander, P. 2001. Biological control of the cassava mealybug in Africa: A review. Biol. Control 21:214–229.
Neuenschwander, P., and Ajuonu, O. 1995. Measuring host finding capacity and arrestment of natural enemies of the cassava mealybug, Phenacoccus manihoti, in the field. Entomol. Exp. Appl. 77:47–55.
Ngi-Song, A. J., and Overholt, W. A. 1997. Host location and acceptance by Cotesia flavipes Cameron and C.sesamiae (Cameron) (Hymenoptera: Braconidae), parasitoids of African gramineous stemborers: Role of frass and other host cues. Biol. Control 9:136–142.
Ngi-Song, A. J., Overholt, W. A., Njagi, P. G. N., Dicke, M., Ayertey, J. N., and Lwande, W. 1996. Volatile infochemicals used in host and host habitat location by Cotesia flavipes Cameron and Cotesia sesamiae (Cameron) (Hymenoptera: Braconidae), larval parasitoids of stemborers on graminae. J. Chem. Ecol. 22:307–323.
Pichersky, E., and Gershenzon, J. 2002. The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Curr. Opin. Plant Biol. 5:237–243.
Röse, U. S. R., Alborn, H. T., Makranczy, G., Lewis, W. J., and Turmlinson, J. H. 1997. Host recognition by the specialist endoparasitoid Microplitis croceipes (Hymenoptera: Braconidae): Role of host and plant-related volatiles. J. Insect Behav. 10:313–330.
Schnee, C., Köllner, T. G., Held, M., Turlings, T. C. J., Gershenzon, J., and Degenhardt, J. 2006. The products of a single maize sesquiterpene synthase form a volatile defense signal that attracts natural enemies of maize herbivores. Proc. Natl. Acad. Sci. USA 103:1129–1134.
Sharma, H. C. 1998. Bionomics, host plant resistance, and management of the legume pod borer, Maruca vitrata—a review. Crop Prot. 17:373–386.
Shimoda, T., Ozawa, R., Sano, K., Yano, E., and Takabayashi, J. 2005. The involvement of volatile infochemicals from spider mites and from food-plants in prey location of the generalist predatory mite Neoseiulus californicus. J. Chem. Ecol. 31:2019–2032.
Steinberg, S., Dicke, M., Vet, L. E. M., and Wanningen, R. 1992. Response of the braconid parasitoid Cotesia (=Apanteles) glomerata to volatile infochemicals: effects of bioassay set-up, parasitoid age and experience and barometric flux. Entomol. Exp. Appl. 63:163–175.
Tamò, C., Ricard, I., Held, M., Davison, A., and Turlings, T. C. J. 2006. A comparison of naïve and conditioned responses of three generalist endoparasitoids of lepidopteran larvae to host-induced plants odours. Anim. Biol. 56:205–220.
Taylor, T. A. 1978. Maruca testulalis, an important pest of tropical grain legumes, pp. 193–200, in S. R. Singh, H. F. van Emden and T. A. Taylor (eds.). Pests of Grain Legumes: Ecology and Control. Academic Press, London.
Turlings, T. C. J., Tumlinson, J. H., and Lewis, W. J. 1990. Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science 250:1251–1253.
Turlings, T. C. J., Tumlinson, J. H., Eller, F. J., and Lewis, W. J. 1991a. Larval-damaged plants: source of volatile synomones that guide the parasitoid Cotesia marginiventris to the micro-habitat of its hosts. Entomol. Exp. Appl. 58:75–83
Turlings, T. C. J., Tumlinson, J. H., Heath, R. R., Proveaux, A. T., and Doolittle, R. 1991b. Isolation and identification of allelochemicals that attract the larval parasitoid Cotesia marginiventris (Cresson), to the microhabitat of one of its hosts. J. Chem. Ecol. 17:2235–2251.
Turlings, T. C. J., Wäckers, F. L., Vet, L. E. M., Lewis, W. J., and Tumlinson, J. H. 1993. Learning of host-finding cues by hymenopterous parasitoids, pp. 51–78, in D. R. Papaj and A. C. Lewis (eds.). Insect Learning: Ecological and Evolutionary Perspectives. Chapman & Hall, New York.
Van Lenteren, J. C. and Manzaroli, G. 1999. Evaluation and use of predators and parasitoids for biological control of pests in greenhouses, pp. 183–201, in R. Albajes, M. L. Gullino, J. C. van Lenteren, and Y. Elad (eds.). Integrated Pest and Disease Management in Greenhouse Crop. Kluwer Publishers, Dordrecht.
Vet, L. E. M. 2001. Parasitoid searching efficiency links behaviour to population processes. Appl. Entomol. Zool. 36(4):399–408.
Vet, L. E. M. and Dicke, M. 1992. Ecology of infochemical use by natural enemies in a tritrophic context. Annu. Rev. Entomol. 37:141–172.
Vet, L. E. M., De Jong, A. G., Franchi, E., and Papaj, D. R. 1998. The effect of complete versus incomplete information on odour discrimination in a parasitic wasp. Anim Behav. 55:1271–1279.
Vinson, S. B. 1976. Host selection by insect parasitoids. Annu. Rev. Entomol. 21:109–134.
Wäckers, F. L. 2004. Assessing the suitability of flowering herbs as parasitoid food sources: flower attractiveness and nectar accessibility. Biol. Control 29:307–314.
Wäckers, F. L., and Lewis, W. J. 1994. Olfactory and visual learning and their combined influence on host site location by the parasitoid Microplitis croceipes (Cresson). Biol. Control 4:105–112.
Wardle, A. R. 1990. Learning of host microhabitat colour by Exeristes roborator (F.) (Hymenoptera: Ichneumonidae). Anim. Behav. 39:914–923.
Wardle, A. R., and Borden, J. H. 1990. Learning of host microhabitat form by Exeristes roborator (F.) (Hymenoptera: Ichneumonidae). J. Insect Behav. 3:251–263.
Wiedenmann, R. N., and Smith, J. W. 1997. Attribute of natural enemies in ephemeral crop habitats. Biol. Control 10:16–22.
Acknowledgements
We thank the Netherlands Universities’ Foundation for International Cooperation (NUFFIC) for financially supporting this work through the Netherlands Fellowship Programmes (NFP). We also thank Mathias Azokpota, Richard Houndafoché, and Basile Dato for technical assistance at the International Institute of Tropical Agriculture (IITA), Benin Station. The comments by two anonymous reviewers were helpful for the improvement of the manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Dannon, E.A., Tamò, M., Van Huis, A. et al. Effects of Volatiles from Maruca vitrata Larvae and Caterpillar-Infested Flowers of Their Host Plant Vigna unguiculata on the Foraging Behavior of the Parasitoid Apanteles taragamae . J Chem Ecol 36, 1083–1091 (2010). https://doi.org/10.1007/s10886-010-9859-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-010-9859-2